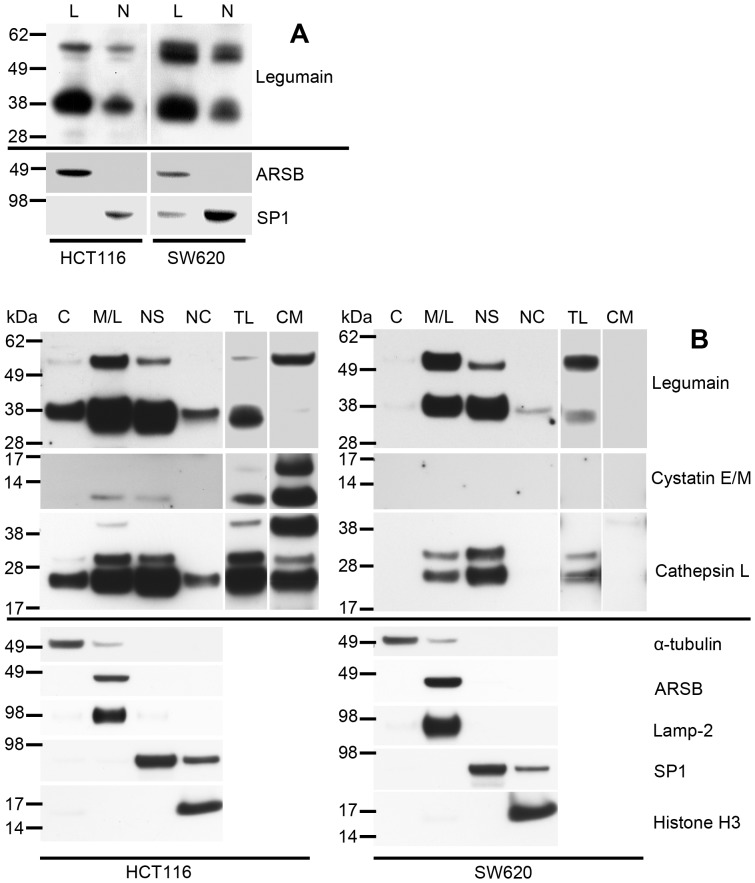
Figure 2. Legumain, cystatin E/M and cathepsin L expressions in subcellular fractions of HCT116 and SW620 cells.
(A) Immunoblots of legumain in lysosomal (L) and nuclear (N) fractions enriched from HCT116 and SW620 cells using density gradient centrifugation. All lanes were loaded with 15 µg total protein from each fraction. Purity controls of the subcellular fractions were assessed by staining for ARSB (soluble lysosomal protein) and SP1 (nuclear transcription factor). (B) Immunoblots of legumain (top panels), cystatin E/M (second panels) and cathepsin L (third panels) in enriched subcellular compartments isolated from HCT116 and SW620 cells using a commercial kit: Cytosol (C), membranes/lysosomes (M/L), nuclear soluble (NS), nuclear chromatin bound (NC), total lysate (TL) and conditioned media (CM). All lanes were loaded with 15 µg total protein from each fraction, except conditioned media where proteins precipitated from 1 ml was loaded. Purity controls of the different subcellular fractions were assessed by staining for α-tubulin (cytosolic protein), ARSB (soluble lysosomal protein), lamp-2 (lysosome membrane-associated protein), SP1 (nuclear transcription factor) and histone H3 (nuclear chromatin bound protein). Uncut immunoblots of legumain and cathepsin L (Fig. S2C).