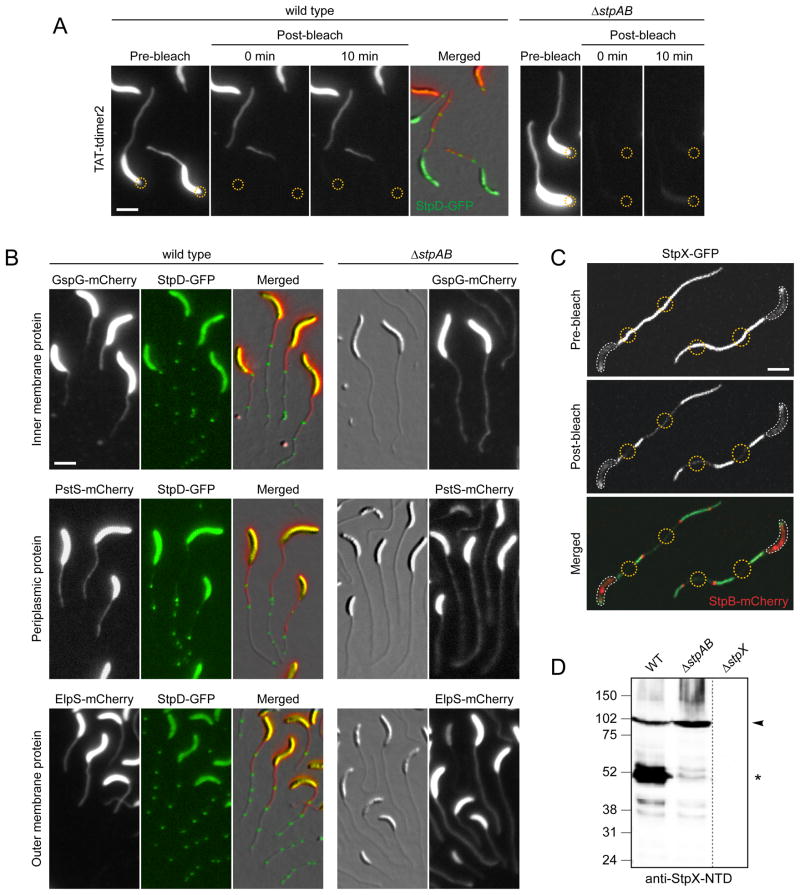
Figure 5. Crossbands serve as protein diffusion barriers.
(A) Analysis of the compartmentalization of soluble and periplasmic red fluorescent protein (tdimer2) using FLIP. Cells of strain SS269 (stpD::stpD-gfp pPxyl-TAT-tdimer2) and SS216 (ΔstpAB pPxyl-TAT-tdimer2) were cultured in M2G−P containing 0.3% xylose for 24 h. Cells were mounted on an agarose pad and exposed to a laser pulse in the regions indicated by a yellow circle. Scale bar: 3 μm.
(B) Crossbands compartmentalize periplasmic, inner- and outer membrane proteins. Cells of strains SS277 (stpD::stpD-gfp Pxyl-gspG-mcherry), SS272 (ΔstpAB Pxyl::Pxyl-gspG-mcherry), SS299 (stpD::stpD-gfp Pxyl::Pxyl-pstS-mcherry), SS302 (ΔstpAB Pxyl::Pxyl-pstS-mcherry), SS283 (stpD::stpD-gfp Pxyl::Pxyl-elpS-mcherry), and SS284 (ΔstpAB Pxyl::Pxyl-elspS-mcherry) were first grown in M2G−P for 36 h and then induced with 0.3% xylose for 11–13 h. Scale bar: 3 μm.
(C) StpX-GFP mobility requires compartmentalization of the stalk from the cell body by the newest crossband. Cells of strain YB5058 (stpX::stpX-gfp Pxyl::Pxyl-stpB-mcherry) were grown in HIGG-30 μM phosphate containing 0.3% xylose and mounted on an agarose pad. First, StpB-mCherry fluorescence was imaged to identify regions of interest (yellow circles). Then, these regions were simultaneously bleached for 50 sec, followed by the acquisition of a post-bleach image. White lines outline the cell bodies. Scale bar: 3 μm.
(D) Crossbands affect the processing of the stalk-specific protein StpX. Wild-type, ΔstpAB (SW51) and ΔstpX (YB5231) cells were grown to stationary phase in HIGG-30 μM phosphate and subjected to immunoblot analysis using an antibody raised against the N-terminal domain of StpX (anti-StpX-NTD). Arrowheads denote the full-length version of StpX, asterisks the dominant short fragment. See also Figure S5.