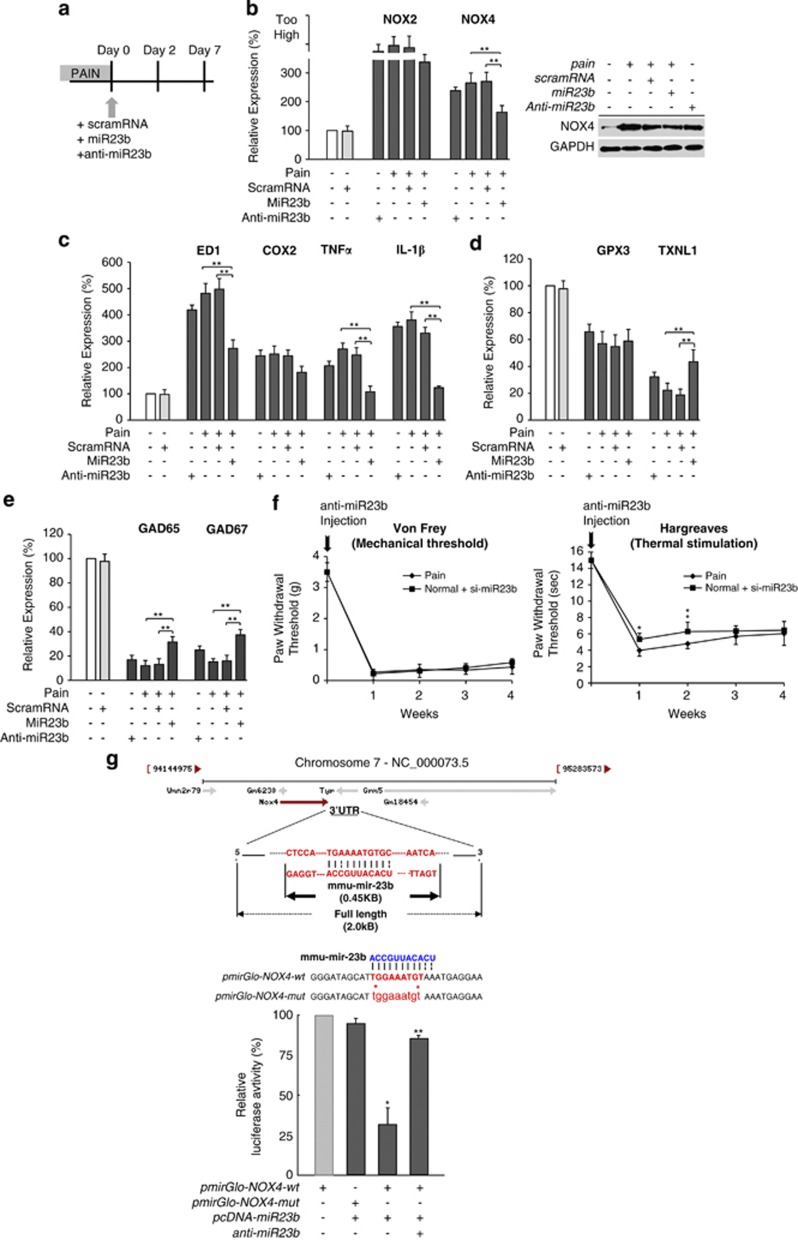
Figure 2.
The intrathecal administration of miR23b and anti-miR23b affects inflammatory factor expression, ROS production, and the expression of redox-scavenging genes. (a) Diagram showing the injection of scramRNA or miR23b with DharmaFECT. (b) RT-PCR and western blot analysis were used to determine the expression levels of ROS-generating proteins after injection of miR23b in the induced neuropathic pain model. Both NOX2 and NOX4 were significantly decreased. In contrast, the injection of anti-miR23b into healthy mice induced ROS production (**P<0.01). (c) MiR23b reduced the expression of the inflammatory factors ED1, COX2, TNF-α, and IL-1β. However, the inflammatory factors were increased when the anti-miR23b was injected (**P<0.01). (d) The expression of the redox-scavenger genes, especially TXNL1, was significantly increased after intrathecal administration of miR23b. In contrast, the expression levels of the redox-scavenger genes, TXNL1, were decreased after injection of anti-miR23b (**P<0.01). (e) Moreover, the expression level of GAD65/67 was also increased compared to animals with neuropathic pain, as shown by RT-PCR. However, suppression of miR23b by injection of anti-miR23b led to a dramatic decrease in GAD65/67 expression (**P<0.01). (f) The PWT was measured using von Frey to determine mechanical hypersensitivity and the Hargreaves test to measure sensitivity to noxious thermal stimulation. After the injection of anti-miR23b, hypersensitivity was significantly increased for 4 weeks, similar to the neuropathic pain model. (*P<0.05; **P<0.01). (g) Genomic localization and structure of 3′-UTR region of miR23b target sequence. Luciferase assay showed that miR23b directly bound the target gene, NOX4 (*P<0.05; **P<0.01)