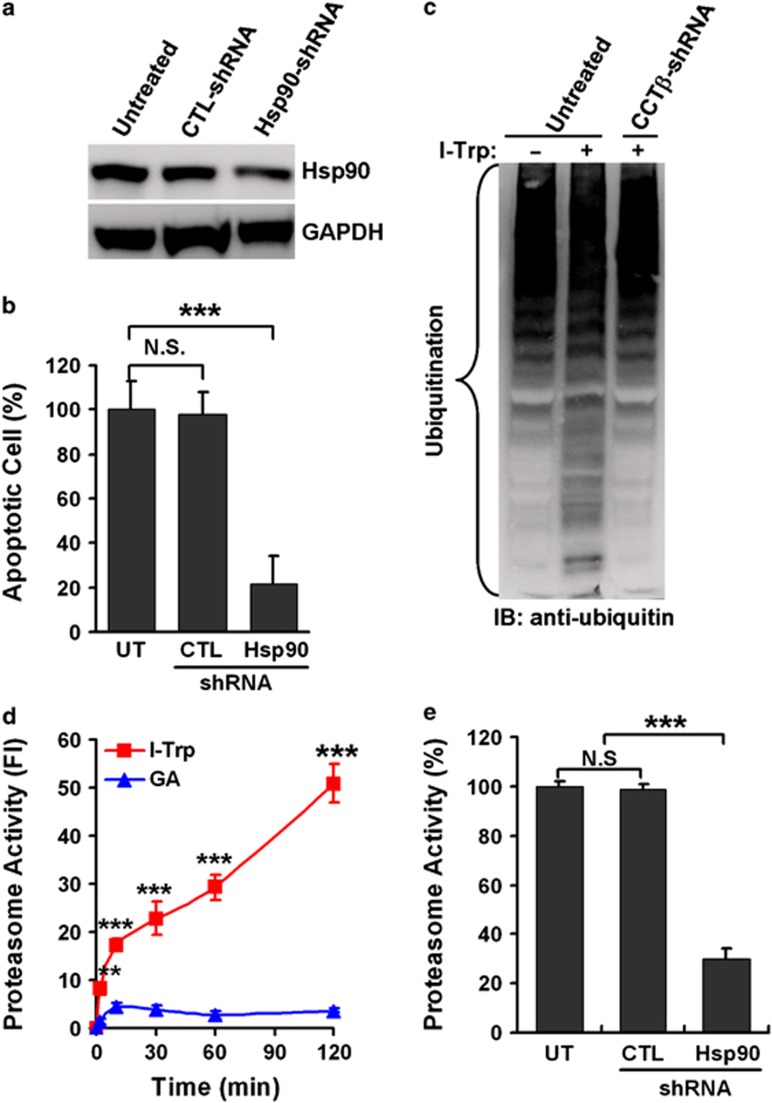
Figure 3.
The activation of Hsp90-associated UPS in response to I-Trp treatment. (a) The protein levels of Hsp90 and glyceraldehyde 3-phosphate dehydrogenase (GAPDH) were determined in cell lysates from HEK-293 cells either untreated or treated with stably expressing control (CTL) or Hsp90 shRNA by western blot analysis using specific antibodies. (b) The knockdown of Hsp90 inhibits I-Trp-induced apoptosis in HEK-293 cells. After treatment with I-Trp (5 μM) for 24 h, cell apoptosis was assessed via PI-based flow cytometric analysis. (c) Destruction of the β-tubulin:CCT-β complex with I-Trp induces intracellular protein ubiquitination. Cell lysates from HEK-293 cells either untreated or treated with stably expressing CCT-β shRNA were collected 24 h after I-Trp (5 μM) treatment and immunoblotted (IB) with anti-ubiquitin antibodies. (d) Targeting the β-tubulin:CCT-β complex with I-Trp incurs Hsp90-associated proteasome activity. HEK-293 cells were treated with either I-Trp (5 μM) or GA (1 μM) for the designated time periods. Cell lysates were examined using the proteasome activity assay. (e) HEK-293 cells either untreated or treated with stably expressing CTL or Hsp90 shRNA were treated with I-Trp (5 μM) for 2 h. Cell lysates were then analyzed for proteasome activity. The results in (b), (d) and (e) are the mean of three independent experiments. The values shown represent the mean±S.D. The symbols ‘**' and ‘***' denote statistical significances at P<0.001 and P<0.01, respectively. NS represents results that are not significant