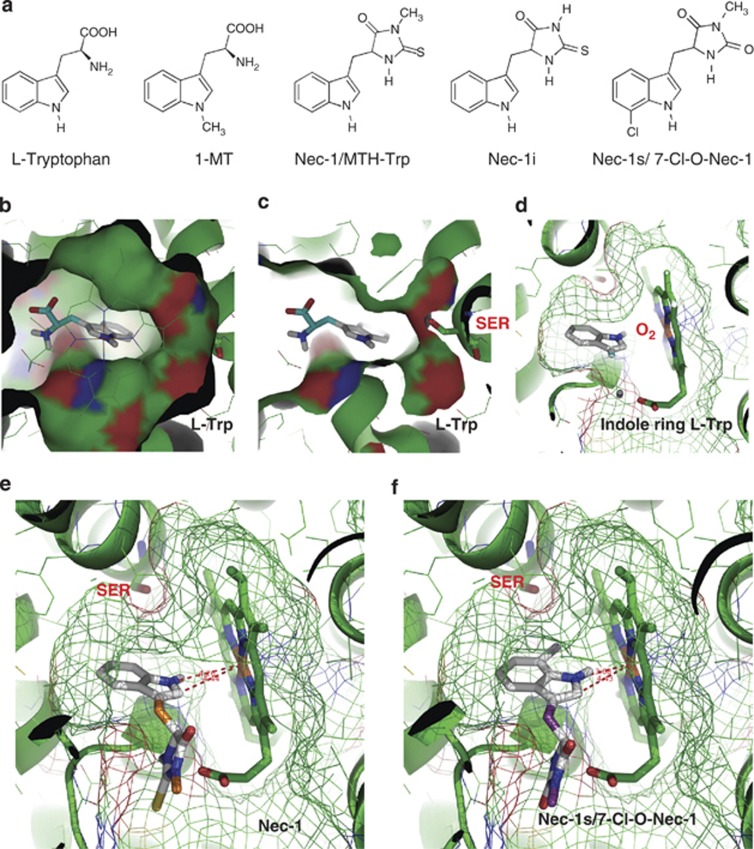
Figure 2.
Molecular docking of substrates, inhibitors and Nec-1 variants on human IDO. (a) Chemical structures of ℒ-Trp, 1-MT, Nec-1/MTH-Trp, Nec-1i and Nec-1s. (b) Surface view of IDO's pocket-like active site, with the docked pose of ℒ-Trp as seen from the enzyme's heme in the foreground. (c) Same viewing position partly surfaced, now showing Ser167 in relation to the ligand's indole ring. (d) Same docking pose of ℒ-Trp (amino-acid moiety clipped off for clarity) now as seen from the pocket entrance with the heme on the right, with meshed surface showing the narrow pocket. The gap between the heme's iron versus positions 1 and 2 of the indole ring leaves sufficient space for the missing iron-bound dioxygen. (e) The docked pose of Nec-1 shows a symmetrical distance between the heme's iron and position 1 or 2 of the indole ring, also allowing sufficient space for dioxygen. (f) For the analogous pose of Nec-1s, the indole ring is slightly tilted due to a steric clash of Ser167 with the indole's 7-Cl substituent. This brings the indole ring substantially closer to the heme, suggesting that this pose no longer leaves sufficient space for molecular oxygen, or that Nec-1s may be unable to fit into the pocket of dioxygen-bound IDO