Abstract
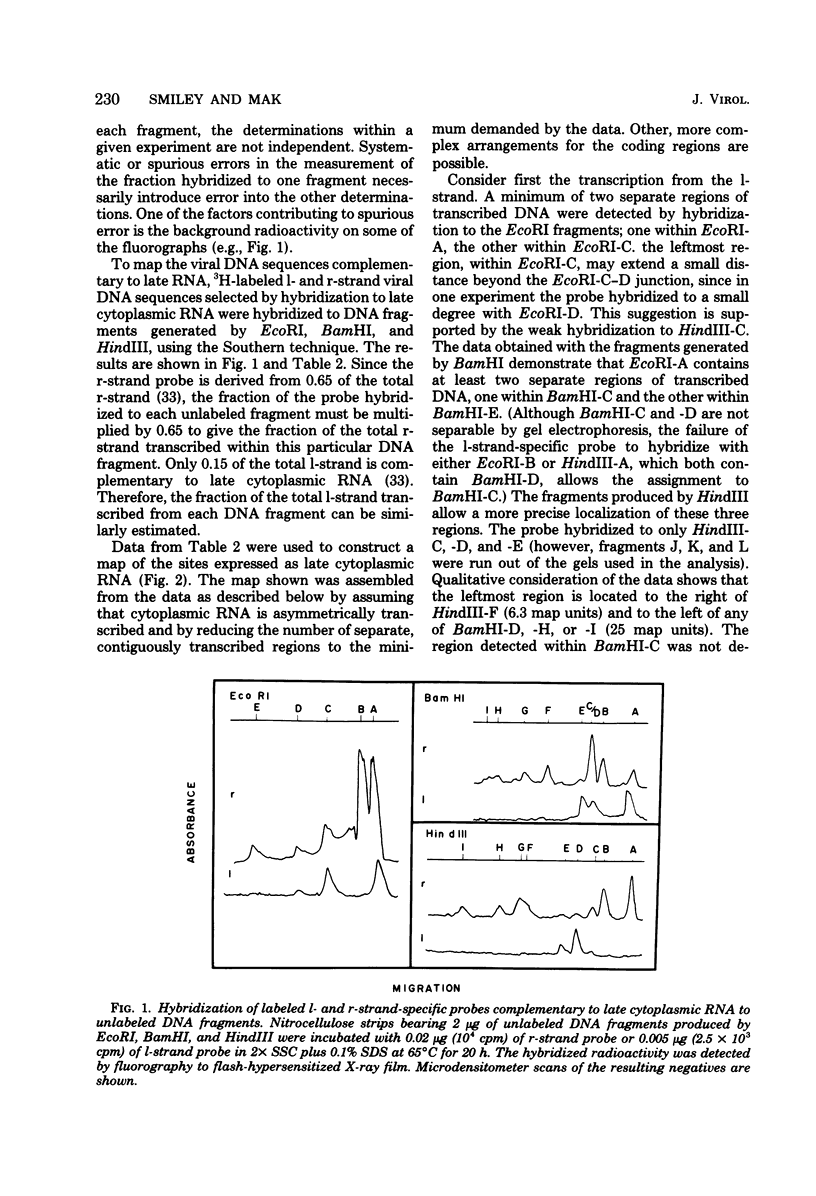
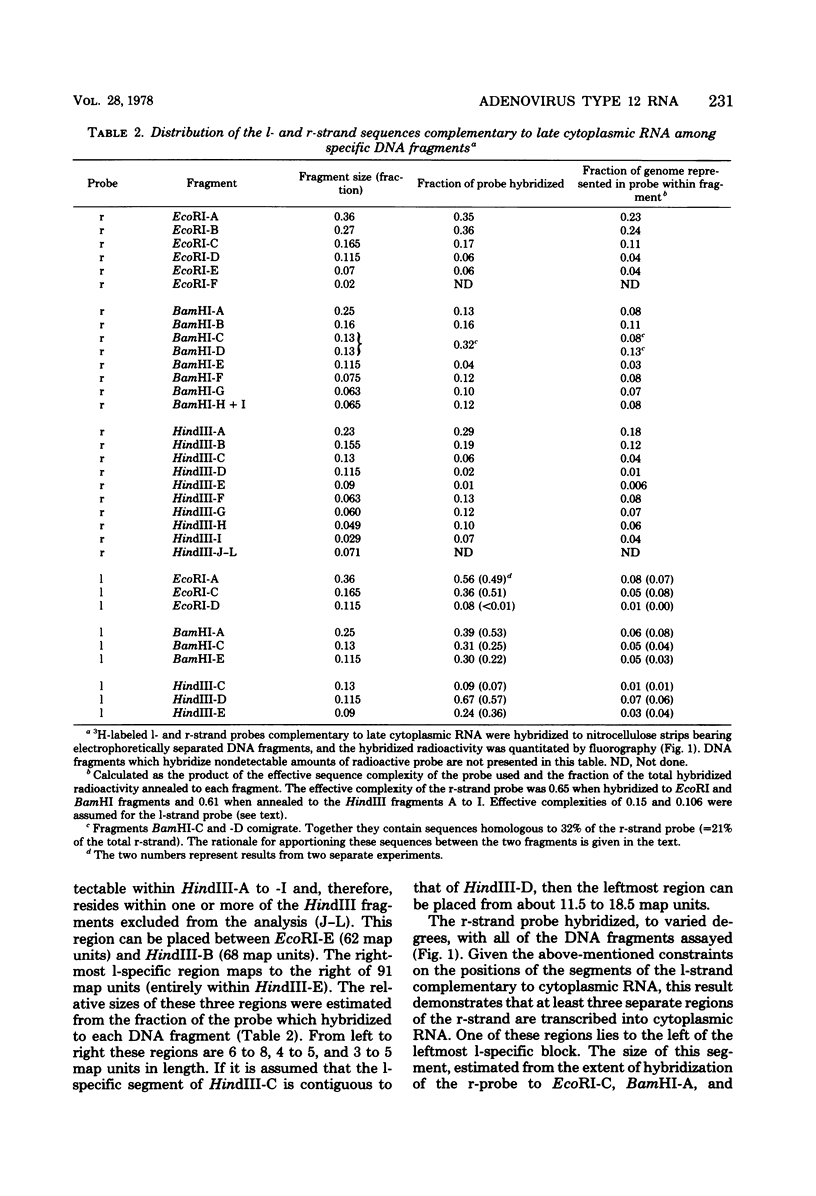
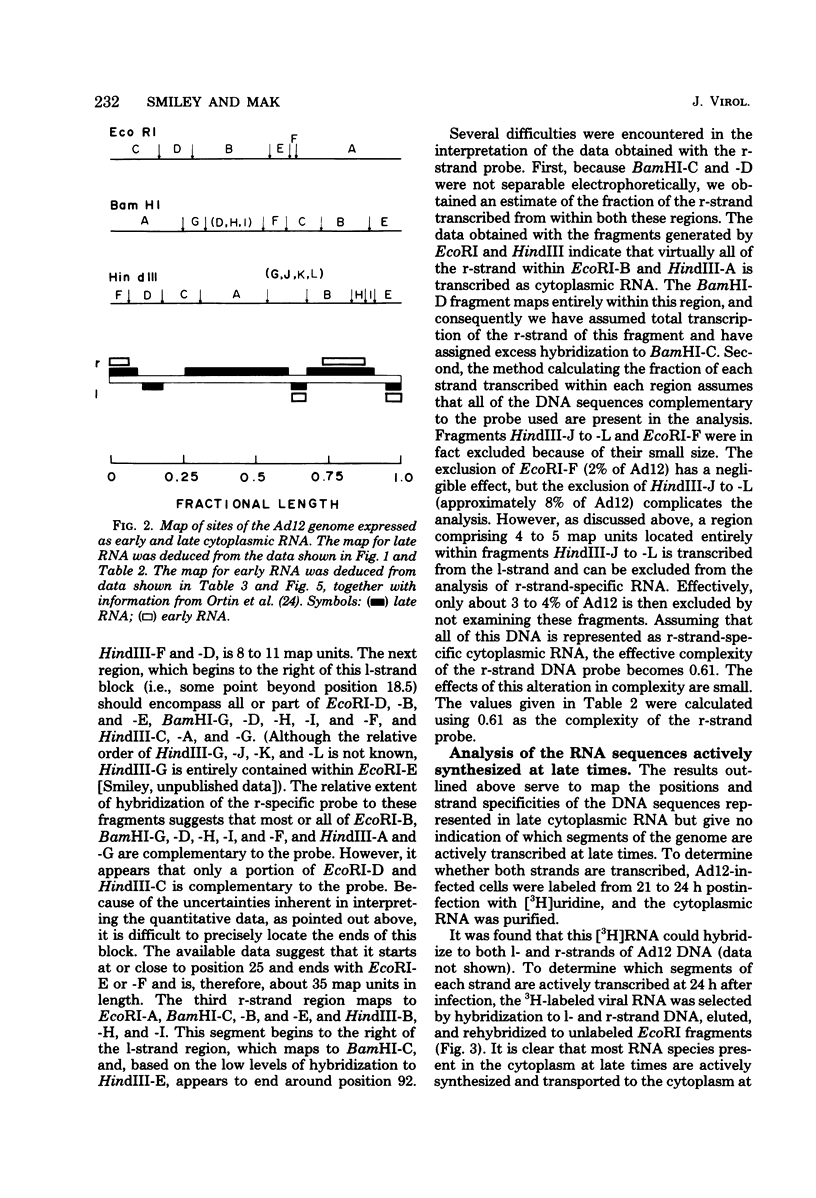
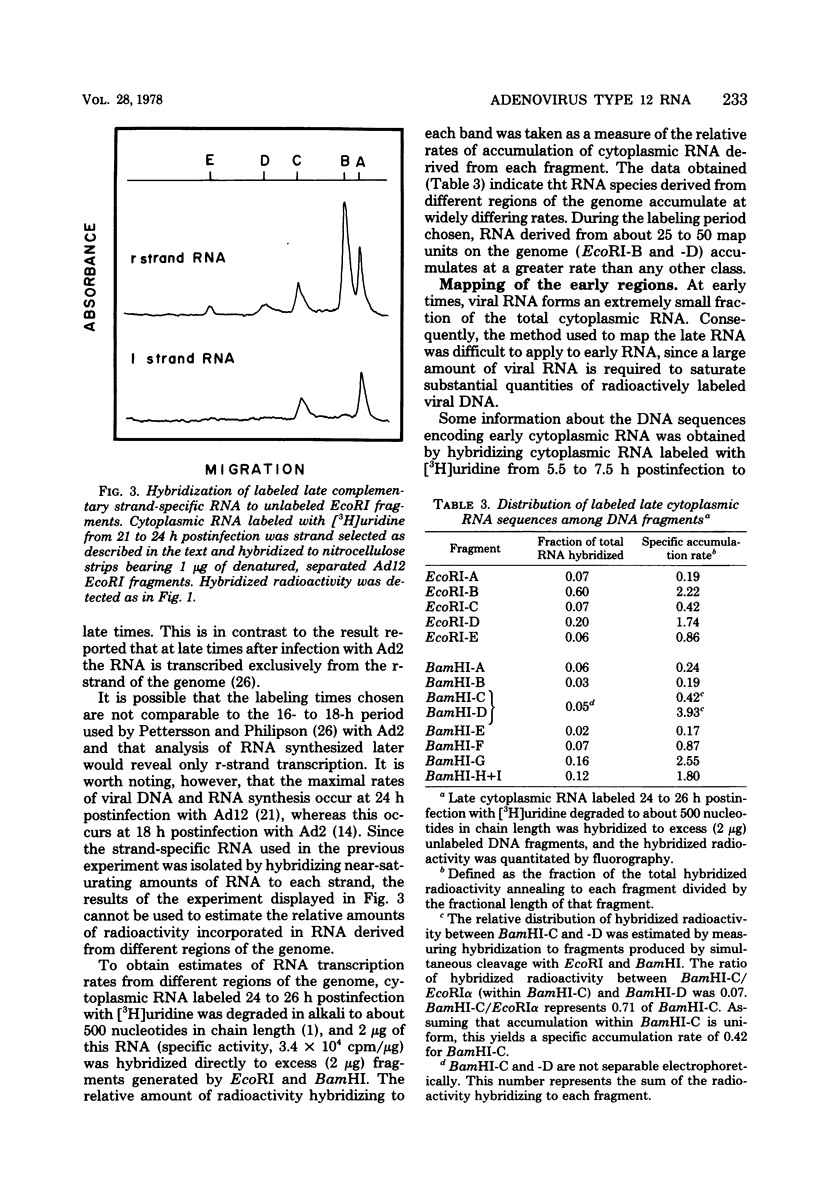
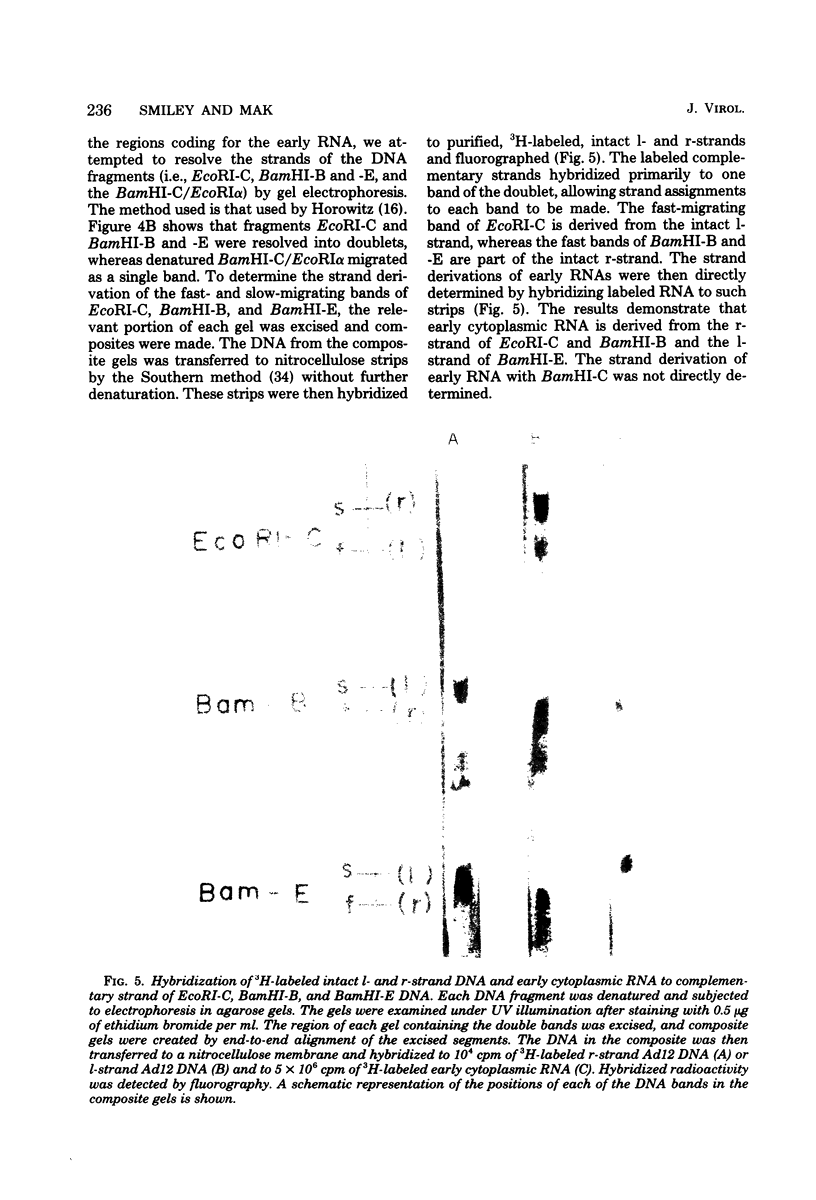
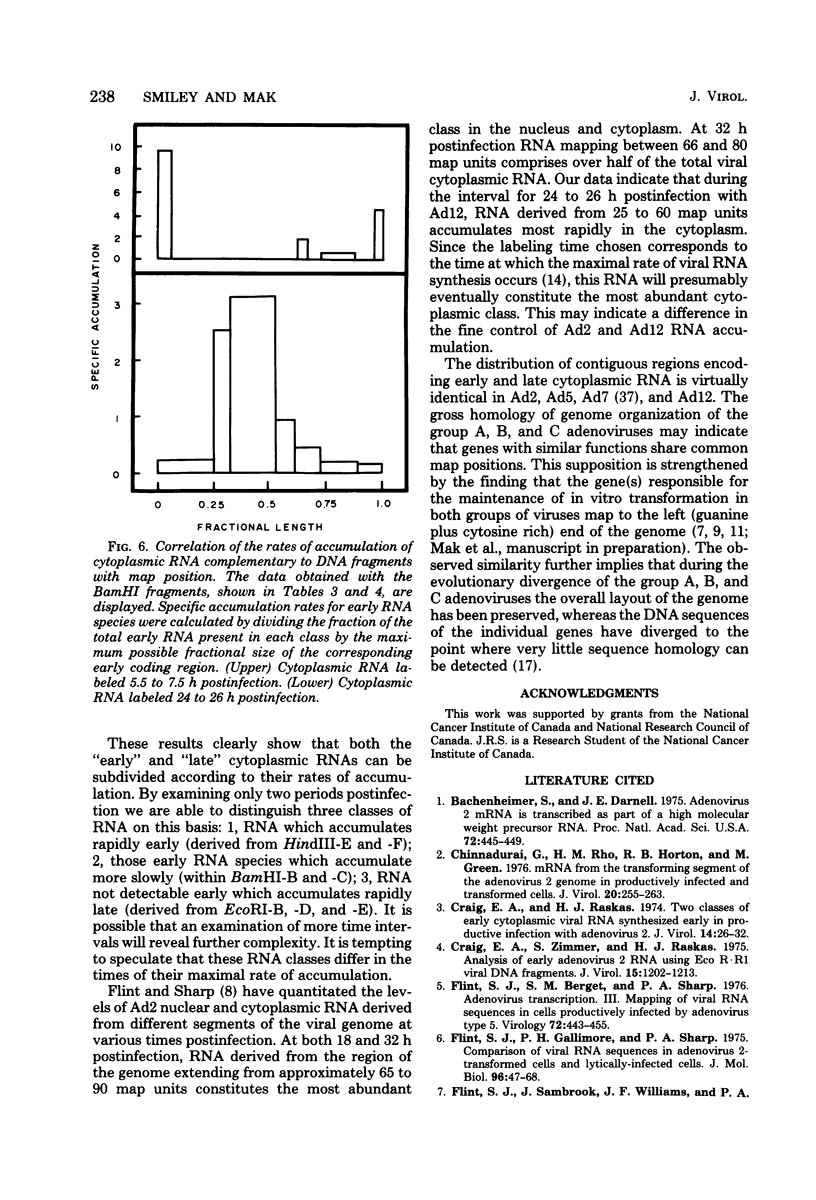
The regions of the adenovirus type 12 genome which encode l- and r-strand-specific cytoplasmic RNA were mapped by the following procedure. Radioactive, intact, separated complementary strands of the viral genome were hybridized to saturating amounts of unlabeled late cytoplasmic RNA. The segments of each DNA strand complementary to the RNA were then purified by S1 nuclease digestion of the hybrids. The arrangement of the coding regions of each strand was deduced from the pattern of hybridization of these probes to unlabeled viral DNA fragments produced by digestion with EcoRI, BamHI, and HindIII.. The resulting map is similar, if not identical, to that of adenovirus type 2. The subset of the late cytoplasmic RNA sequences which are expressed at early times were located on the map by hybridizing labeled, early cytoplasmic RNA to both unlabeled DNA fragments and unlabeled complementary strands of specific fragments. Early cytoplasmic RNA hybridized to the r-strand to EcoRI-C and BamHI-B and to the l-strand of BamHI-E. Hybridization to BamHI-C was also observed. The relative rates of accumulation of cytoplasmic RNA complementary to individual restriction fragments was measured at both early and late times. Early during infection, most of the viral RNA appearing in the cytoplasm was derived from the molecular ends of the genome. Later (24 to 26 h postinfection) the majority of the newly labeled cytoplasmic RNA was transcribed from DNA sequences mapping between 25 and 60 map units on the genome.
Full text
PDF





Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Chinnadurai G., Rho H. M., Horton R. B., Green M. mRNA from the transforming segment of the adenovirus 2 genome in productively infected and transformed cells. J Virol. 1976 Oct;20(1):255–263. doi: 10.1128/jvi.20.1.255-263.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Craig E. A. Analysis of early adenovirus 2 RNA using Eco R-R1 viral DNA fragments. J Virol. 1975 May;15(5):1202–1213. doi: 10.1128/jvi.15.5.1202-1213.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Craig E. A., Raskas H. J. Effect of cycloheximide on RNA metabolism early in productive infection with adenovirus 2. J Virol. 1974 Jul;14(1):26–32. doi: 10.1128/jvi.14.1.26-32.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flint S. J., Berget S. M., Sharp P. A. Adenovirus transcription. III. Mapping of viral RNA sequences in cells productively infected by adenovirus type 5. Virology. 1976 Jul 15;72(2):443–455. doi: 10.1016/0042-6822(76)90173-2. [DOI] [PubMed] [Google Scholar]
- Flint S. J., Gallimore P. H., Sharp P. A. Comparison of viral RNA sequences in adenovirus 2-transformed and lytically infected cells. J Mol Biol. 1975 Jul 25;96(1):47–68. doi: 10.1016/0022-2836(75)90181-3. [DOI] [PubMed] [Google Scholar]
- Flint S. J., Sambrook J., Williams J. F., Sharp P. A. Viral nucleic acid sequences in transformed cells. IV. A study of the sequences of adenovirus 5 DNA and RNA in four lines of adenovirus 5-transformed rodent cells using specific fragments of the viral genome. Virology. 1976 Jul 15;72(2):456–470. doi: 10.1016/0042-6822(76)90174-4. [DOI] [PubMed] [Google Scholar]
- Flint S. J., Sharp P. A. Adenovirus transcription. V. Quantitation of viral RNA sequences in adenovirus 2-infected and transformed cells. J Mol Biol. 1976 Sep 25;106(3):749–774. doi: 10.1016/0022-2836(76)90263-1. [DOI] [PubMed] [Google Scholar]
- GREEN M., PINA M. BIOCHEMICAL STUDIES ON ADENOVIRUS MULTIPLICATION, VI. PROPERTIES OF HIGHLY PURIFIED TUMORIGENIC HUMAN ADENOVIRUSES AND THEIR DNA. Proc Natl Acad Sci U S A. 1964 Jun;51:1251–1259. doi: 10.1073/pnas.51.6.1251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- GREEN M., PINA M. Biochemical studies on adenovirus multiplication. IV. Isolation, purification, and chemical analysis of adenovirus. Virology. 1963 May;20:199–207. doi: 10.1016/0042-6822(63)90157-0. [DOI] [PubMed] [Google Scholar]
- Gallimore P. H. Viral DNA in transformed cells. II. A study of the sequences of adenovirus 2 DNA IN NINE LINES OF TRANSFORMED RAT CELLS USING SPECIFIC FRAGMENTS OF THE VIRAL GENOME;. J Mol Biol. 1974 Oct 15;89(1):49–72. doi: 10.1016/0022-2836(74)90162-4. [DOI] [PubMed] [Google Scholar]
- Garon C. F., Berry K. W., Hierholzer J. C., Rose J. A. Mapping of base sequence heterologies between genomes from different adenovirus serotypes. Virology. 1973 Aug;54(2):414–426. doi: 10.1016/0042-6822(73)90153-0. [DOI] [PubMed] [Google Scholar]
- Graham F. L., Abrahams P. J., Mulder C., Heijneker H. L., Warnaar S. O., De Vries F. A., Fiers W., Van Der Eb A. J. Studies on in vitro transformation by DNA and DNA fragments of human adenoviruses and simian virus 40. Cold Spring Harb Symp Quant Biol. 1975;39(Pt 1):637–650. doi: 10.1101/sqb.1974.039.01.077. [DOI] [PubMed] [Google Scholar]
- Hayward G. S. Gel electrophoretic separation of the complementary strands of bacteriophage DNA. Virology. 1972 Jul;49(1):342–344. doi: 10.1016/s0042-6822(72)80042-4. [DOI] [PubMed] [Google Scholar]
- Horwitz M. S. Bidirectional replication of adenovirus type 2 DNA. J Virol. 1976 Apr;18(1):307–315. doi: 10.1128/jvi.18.1.307-315.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LACY S., GREEN M. BIOCHEMICAL STUDIES ON ADENOVIRUS MULTIPLICATION. VII. HOMOLOGY BETWEEN DNA'S OF TUMORIGENIC AND NONTUMORIGENIC HUMAN ADENOVIRUSES. Proc Natl Acad Sci U S A. 1964 Oct;52:1053–1059. doi: 10.1073/pnas.52.4.1053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Landgraf-Leurs M., Green M. Adenovirus DNA. 3. Separation of the complementary strands of adenovirus types 2, 7 and 12 DNA molecules. J Mol Biol. 1971 Aug 28;60(1):185–202. doi: 10.1016/0022-2836(71)90457-8. [DOI] [PubMed] [Google Scholar]
- Laskey R. A., Mills A. D. Quantitative film detection of 3H and 14C in polyacrylamide gels by fluorography. Eur J Biochem. 1975 Aug 15;56(2):335–341. doi: 10.1111/j.1432-1033.1975.tb02238.x. [DOI] [PubMed] [Google Scholar]
- Lewis J. B., Atkins J. F., Anderson C. W., Baum P. R., Gesteland R. F. Mapping of late adenovirus genes by cell-free translation of RNA selected by hybridization to specific DNA fragments. Proc Natl Acad Sci U S A. 1975 Apr;72(4):1344–1348. doi: 10.1073/pnas.72.4.1344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mak S. Transcription and replication of viral deoxyribonucleic acid in cells coinfected with adenovirus types 2 and 12. J Virol. 1969 Nov;4(5):651–656. doi: 10.1128/jvi.4.5.651-656.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mulder C., Sharp P. A., Delius H., Pettersson U. Specific fragmentation of DNA of adenovirus serotypes 3, 5, 7, and 12, and adeno-simian virus 40 hybrid virus Ad2+ND1 by restriction endonuclease R.EcoRI. J Virol. 1974 Jul;14(1):68–77. doi: 10.1128/jvi.14.1.68-77.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ortin J., Scheidtmann K. H., Greenberg R., Westphal M., Doerfler W. Transcription of the genome of adenovirus type 12. III. Maps of stable RNA from productively infected human cells and abortively infected and transformed hamster cells. J Virol. 1976 Nov;20(2):355–372. doi: 10.1128/jvi.20.2.355-372.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- PEREIRA M. S., MACCALLUM F. O. INFECTION WITH ADENOVIRUS TYPE 12. Lancet. 1964 Jan 25;1(7326):198–199. doi: 10.1016/s0140-6736(64)92290-1. [DOI] [PubMed] [Google Scholar]
- Pettersson U., Mulder C., Deluis H., Sharp P. A. Cleavage of adenovirus type 2 DNA into six unique fragments by endonuclease R-RI. Proc Natl Acad Sci U S A. 1973 Jan;70(1):200–204. doi: 10.1073/pnas.70.1.200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pettersson U., Philipson L. Synthesis of complementary RNA sequences during productive adenovirus infection. Proc Natl Acad Sci U S A. 1974 Dec;71(12):4887–4891. doi: 10.1073/pnas.71.12.4887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pettersson U., Tibbetts C., Philipson L. Hybridization maps of early and late messenger RNA sequences on the adenovirus type 2 genome. J Mol Biol. 1976 Mar 15;101(4):479–501. doi: 10.1016/0022-2836(76)90241-2. [DOI] [PubMed] [Google Scholar]
- Philipson L., Pettersson U., Lindberg U., Tibbetts C., Vennström B., Persson T. RNA synthesis and processing in adenovirus-infected cells. Cold Spring Harb Symp Quant Biol. 1975;39(Pt 1):447–456. doi: 10.1101/sqb.1974.039.01.057. [DOI] [PubMed] [Google Scholar]
- Scheidtmann K. H., Doerfler W. Transcription of the genome of adenovirus type 12. IV. Maps of stable late RNA from productively infected human cells. J Virol. 1977 Jun;22(3):585–590. doi: 10.1128/jvi.22.3.585-590.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharp P. A., Flint S. J. Adenovirus transcription. Curr Top Microbiol Immunol. 1976;74:137–166. doi: 10.1007/978-3-642-66336-9_5. [DOI] [PubMed] [Google Scholar]
- Sharp P. A., Gallimore P. H., Flint S. J. Mapping of adenovirus 2 RNA sequences in lytically infected cells and transformed cell lines. Cold Spring Harb Symp Quant Biol. 1975;39(Pt 1):457–474. doi: 10.1101/sqb.1974.039.01.058. [DOI] [PubMed] [Google Scholar]
- Sharp P. A., Sugden B., Sambrook J. Detection of two restriction endonuclease activities in Haemophilus parainfluenzae using analytical agarose--ethidium bromide electrophoresis. Biochemistry. 1973 Jul 31;12(16):3055–3063. doi: 10.1021/bi00740a018. [DOI] [PubMed] [Google Scholar]
- Smiley J. R., Mak S. Adenovirus type 12-specific RNA sequences during productive infection of KB cells. J Virol. 1976 Jul;19(1):36–42. doi: 10.1128/jvi.19.1.36-42.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Southern E. M. Detection of specific sequences among DNA fragments separated by gel electrophoresis. J Mol Biol. 1975 Nov 5;98(3):503–517. doi: 10.1016/s0022-2836(75)80083-0. [DOI] [PubMed] [Google Scholar]
- Thomas D. C., Green M. Biochemical studies on adenovirus multiplication. XV. Transcription of the adenovirus type II genome during productive infection. Virology. 1969 Oct;39(2):205–210. doi: 10.1016/0042-6822(69)90040-3. [DOI] [PubMed] [Google Scholar]
- Thuring R. W., Sanders J. P., Borst P. A freeze-squeeze method for recovering long DNA from agarose gels. Anal Biochem. 1975 May 26;66(1):213–220. doi: 10.1016/0003-2697(75)90739-3. [DOI] [PubMed] [Google Scholar]
- Tibbetts C., Johansson K., Philipson L. Hydroxyapatite chromatography and formamide denaturation of adenovirus DNA. J Virol. 1973 Aug;12(2):218–225. doi: 10.1128/jvi.12.2.218-225.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tibbetts C., Pettersson U. Complementary strand-specific sequences from unique fragments of adenovirus type 2 DNA for hybridization-mapping experiments. J Mol Biol. 1974 Oct 5;88(4):767–784. doi: 10.1016/0022-2836(74)90398-2. [DOI] [PubMed] [Google Scholar]
- Tibbetts C. Physical organization of subgroup B human adenovirus genomes. J Virol. 1977 Nov;24(2):564–579. doi: 10.1128/jvi.24.2.564-579.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]