Abstract
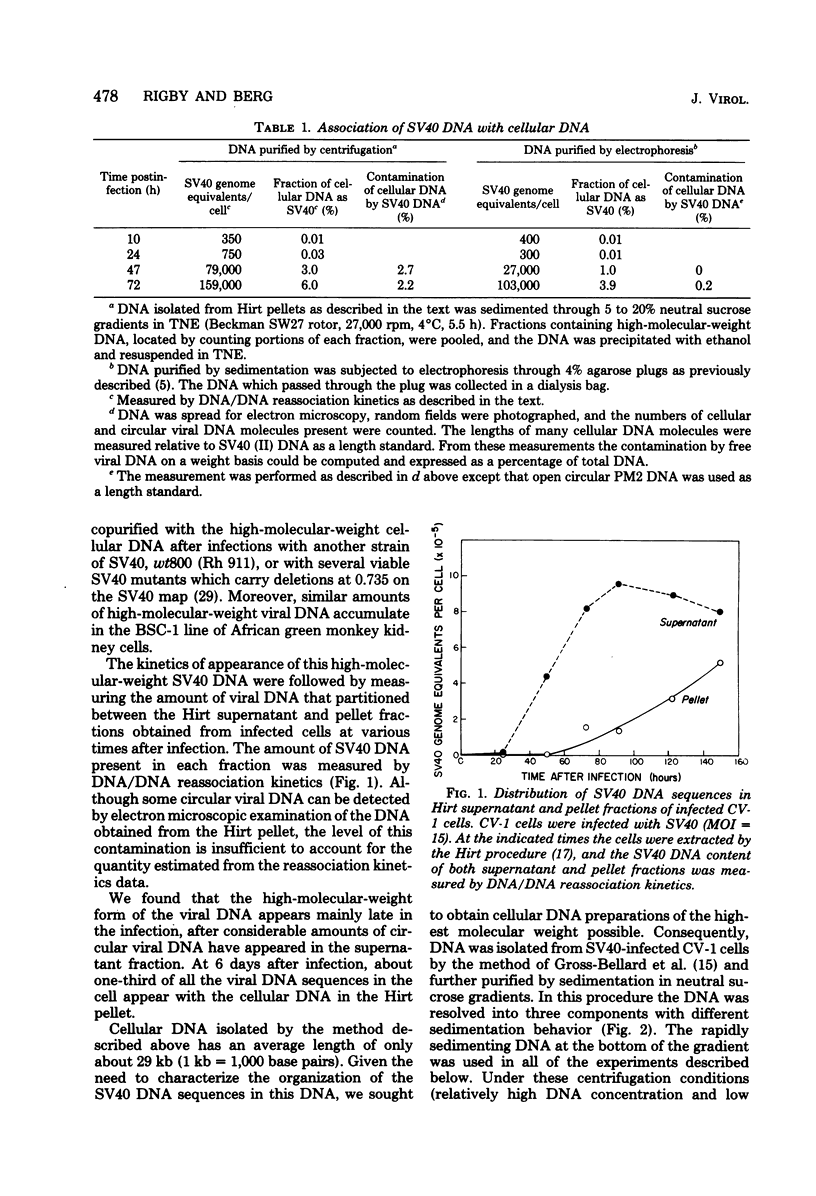
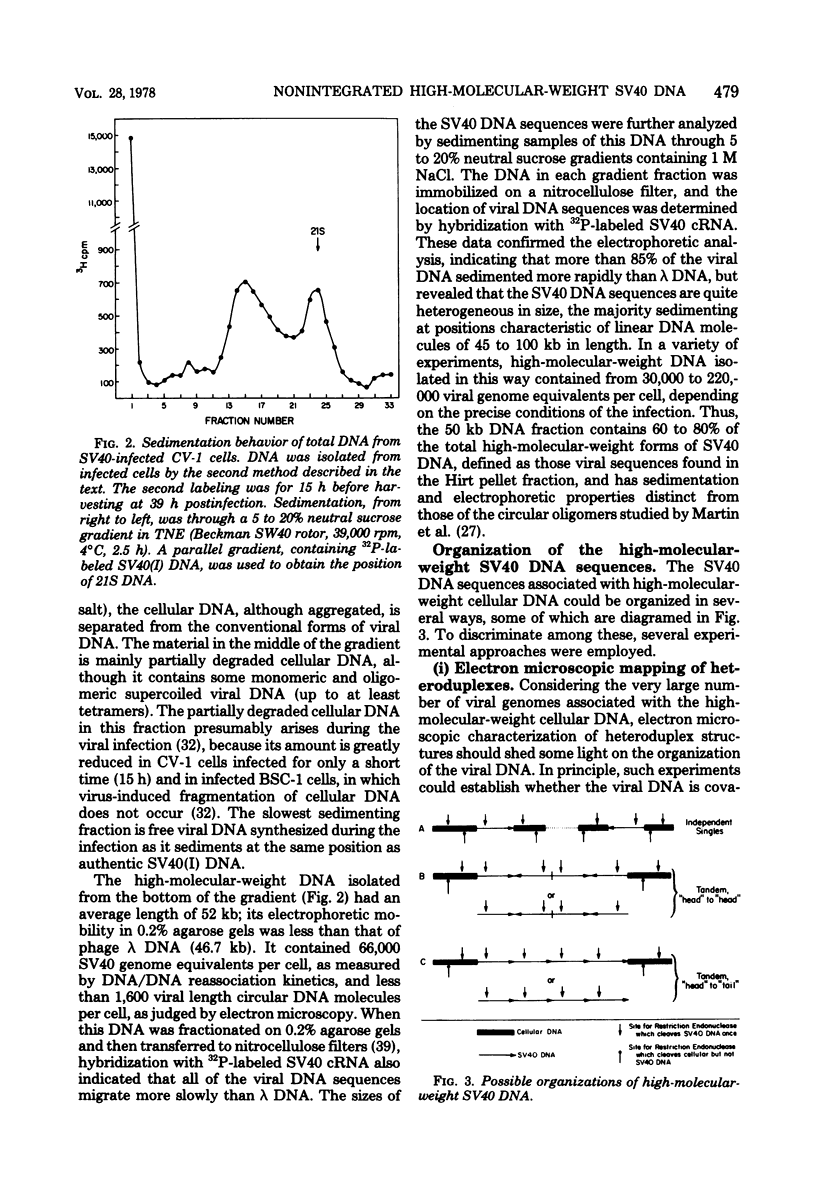
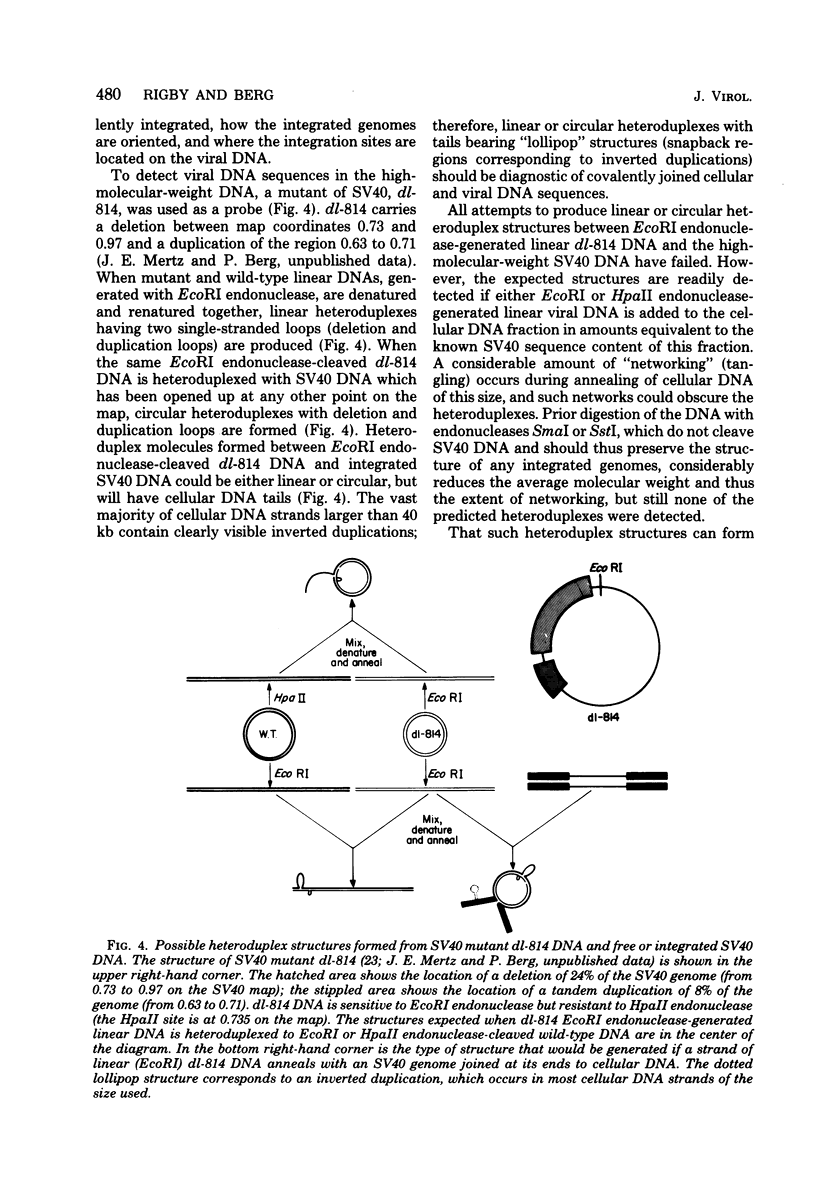
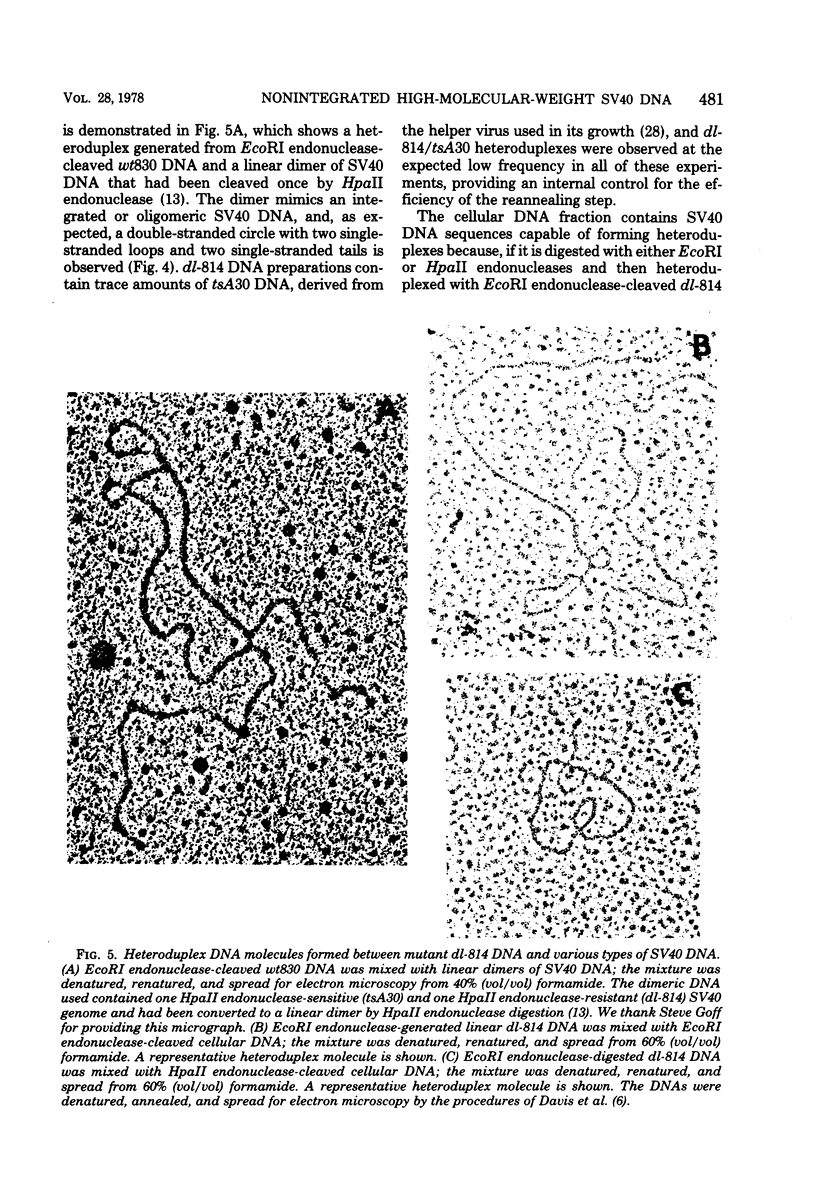
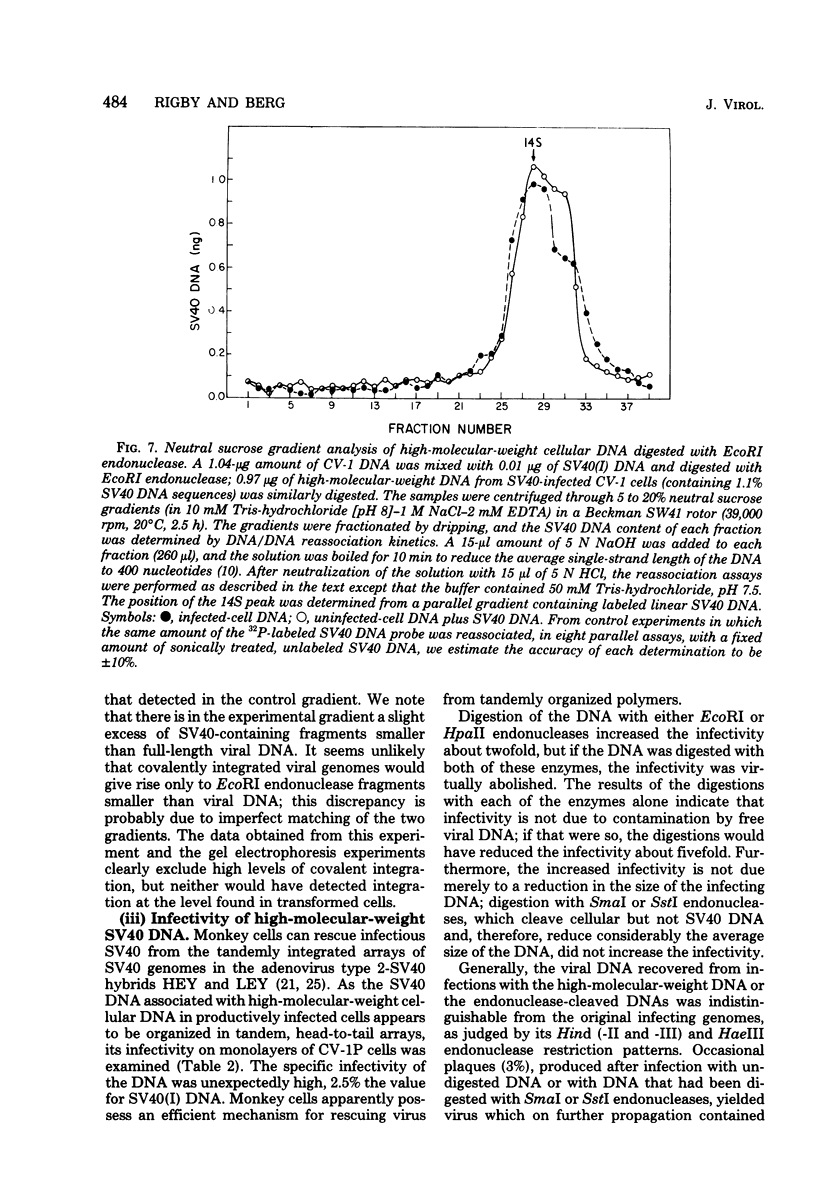
Late after infection of permissive monkey cells by simian virus 40 (SV40), large amounts of SV40 DNA (30,000 to 220,000 viral genome equivalents per cell) can be isolated with the high-molecular-weight fraction of cellular DNA. Hirai and Defendi (J. Virol.9:705-707, 1972) and Hölzel and Sokol (J. Mol. Biol. 84:423-444, 1974) suggested that this SV40 DNA is covalently integrated into the cellular DNA. However, our data indicate that the high-molecular-weight viral DNA is composed of tandem, "head-to-tail" repeats of SV40 DNA and that very little, if any, of this viral DNA is covalently joined to the cellular DNA. This was deduced from the following experimental findings. The size of the SV40 DNA associated with the high-molecular-weight cellular DNA fraction is greater than 45 kilobases, based on its electrophoretic mobility in agarose gels. In this form the SV40 DNA did not produce heteroduplex structures with a marker viral DNA (an SV40 genome with a characteristic deletion and duplication). After the high-molecular-weight DNA was digested with EcoRI or HpaII endonucleases, enzymes which cleave SV40 DNA once, more than 95% of the SV40 DNA migrated as unit-length linear molecules and, after hybridization with the marker viral DNA, the expected heteroduplex structures were easily detected. Digestion of the high-molecular-weight DNA fraction with restriction endonucleases that cleave cellular, but not SV40. DNA did not alter the electrophoretic mobility of the polymeric SV40 DNA, nor did it give rise to molecules that form heteroduplex structures with the marker viral DNA. Polymeric SV40 DNA molecules produced after coinfection by two physically distinguishable SV40 genomes contain only a single type of genome, suggesting that they arise by replication rather than by recombination. The polymeric form of SV40 DNA is highly infectious for CV-1P monolayers (6.5 X 10(4) PFU per microgram of SV40 DNA), yielding virtually exclusively normal, covalently closed circular, monomer-length DNA. Quite clearly these cells have an efficient mechanism for generating monomeric viral DNA from the SV40 DNA polymers.
Full text
PDF









Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Aaij C., Borst P. The gel electrophoresis of DNA. Biochim Biophys Acta. 1972 May 10;269(2):192–200. doi: 10.1016/0005-2787(72)90426-1. [DOI] [PubMed] [Google Scholar]
- Botchan M., Ozanne B., Sugden B., Sharp P. A., Sambrook J. Viral DNA in transformed cells. III. The amounts of different regions of the SV40 genome present in a line of transformed mouse cells. Proc Natl Acad Sci U S A. 1974 Oct;71(10):4183–4187. doi: 10.1073/pnas.71.10.4183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Botchan M., Topp W., Sambrook J. The arrangement of simian virus 40 sequences in the DNA of transformed cells. Cell. 1976 Oct;9(2):269–287. doi: 10.1016/0092-8674(76)90118-5. [DOI] [PubMed] [Google Scholar]
- Burgess R. R., Jendrisak J. J. A procedure for the rapid, large-scall purification of Escherichia coli DNA-dependent RNA polymerase involving Polymin P precipitation and DNA-cellulose chromatography. Biochemistry. 1975 Oct 21;14(21):4634–4638. doi: 10.1021/bi00692a011. [DOI] [PubMed] [Google Scholar]
- Carbon J., Shenk T. E., Berg P. Biochemical procedure for production of small deletions in simian virus 40 DNA. Proc Natl Acad Sci U S A. 1975 Apr;72(4):1392–1396. doi: 10.1073/pnas.72.4.1392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eisen H., Pereira da Silva L., Jacob F. The regulation and mechanism of DNA synthesis in bacteriophage lambda. Cold Spring Harb Symp Quant Biol. 1968;33:755–764. doi: 10.1101/sqb.1968.033.01.086. [DOI] [PubMed] [Google Scholar]
- Enquist L. W., Skalka A. Replication of bacteriophage lambda DNA dependent on the function of host and viral genes. I. Interaction of red, gam and rec. J Mol Biol. 1973 Apr 5;75(2):185–212. doi: 10.1016/0022-2836(73)90016-8. [DOI] [PubMed] [Google Scholar]
- Estes M. K., Huang E. S., Pagano J. S. Structural polypeptides of simian virus 40. J Virol. 1971 May;7(5):635–641. doi: 10.1128/jvi.7.5.635-641.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gallimore P. H. Viral DNA in transformed cells. II. A study of the sequences of adenovirus 2 DNA IN NINE LINES OF TRANSFORMED RAT CELLS USING SPECIFIC FRAGMENTS OF THE VIRAL GENOME;. J Mol Biol. 1974 Oct 15;89(1):49–72. doi: 10.1016/0022-2836(74)90162-4. [DOI] [PubMed] [Google Scholar]
- Gelb L. D., Kohne D. E., Martin M. A. Quantitation of Simian virus 40 sequences in African green monkey, mouse and virus-transformed cell genomes. J Mol Biol. 1971 Apr 14;57(1):129–145. doi: 10.1016/0022-2836(71)90123-9. [DOI] [PubMed] [Google Scholar]
- Gilbert W., Dressler D. DNA replication: the rolling circle model. Cold Spring Harb Symp Quant Biol. 1968;33:473–484. doi: 10.1101/sqb.1968.033.01.055. [DOI] [PubMed] [Google Scholar]
- Goff S. P., Berg P. Structure and formation of circular dimers of simian virus 40 DNA. J Virol. 1977 Oct;24(1):295–302. doi: 10.1128/jvi.24.1.295-302.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gross-Bellard M., Oudet P., Chambon P. Isolation of high-molecular-weight DNA from mammalian cells. Eur J Biochem. 1973 Jul 2;36(1):32–38. doi: 10.1111/j.1432-1033.1973.tb02881.x. [DOI] [PubMed] [Google Scholar]
- Hirai K., Defendi V. Integration of simian virus 40 deoxyribonucleic acid into the deoxyribonucleic acid of permissive monkey kidney cells. J Virol. 1972 Apr;9(4):705–707. doi: 10.1128/jvi.9.4.705-707.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirt B. Selective extraction of polyoma DNA from infected mouse cell cultures. J Mol Biol. 1967 Jun 14;26(2):365–369. doi: 10.1016/0022-2836(67)90307-5. [DOI] [PubMed] [Google Scholar]
- Hölzel F., Sokol F. Integration of progeny simian virus 40 DNA into the host cell genome. J Mol Biol. 1974 Apr 15;84(3):423–444. doi: 10.1016/0022-2836(74)90450-1. [DOI] [PubMed] [Google Scholar]
- Jaenisch R., Levine A. J. Infection of primary African green monkey cells with SV40 monomeric and dimeric DNA. J Mol Biol. 1971 Nov 14;61(3):735–738. doi: 10.1016/0022-2836(71)90076-3. [DOI] [PubMed] [Google Scholar]
- Jovin T. M., Englund P. T., Bertsch L. L. Enzymatic synthesis of deoxyribonucleic acid. XXVI. Physical and chemical studies of a homogeneous deoxyribonucleic acid polymerase. J Biol Chem. 1969 Jun 10;244(11):2996–3008. [PubMed] [Google Scholar]
- Kelly T. J., Jr, Lewis A. M., Jr, Levine A. S., Siegel S. Structure of two adenovirus-simian virus 40 hybrids which contain the entire SV40 genome. J Mol Biol. 1974 Oct 15;89(1):113–126. doi: 10.1016/0022-2836(74)90165-x. [DOI] [PubMed] [Google Scholar]
- Ketner G., Kelly T. J., Jr Integrated simian virus 40 sequences in transformed cell DNA: analysis using restriction endonucleases. Proc Natl Acad Sci U S A. 1976 Apr;73(4):1102–1106. doi: 10.1073/pnas.73.4.1102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laskey R. A., Mills A. D. Enhanced autoradiographic detection of 32P and 125I using intensifying screens and hypersensitized film. FEBS Lett. 1977 Oct 15;82(2):314–316. doi: 10.1016/0014-5793(77)80609-1. [DOI] [PubMed] [Google Scholar]
- Lavi S., Winocour E. Acquisition of sequences homologous to host deoxyribonucleic acid by closed circular simian virus 40 deoxyribonucleic acid. J Virol. 1972 Feb;9(2):309–316. doi: 10.1128/jvi.9.2.309-316.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lewis A. M., Jr, Rowe W. P. Isolation of two plaque variants from the adenovirus type 2-simian virus 40 hybrid population which differ in their efficiency in yielding simian virus 40. J Virol. 1970 Apr;5(4):413–420. doi: 10.1128/jvi.5.4.413-420.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manteuil S., Girard M. Inhibitors of DNA synthesis: their influence on replication and transcription of simian virus 40 DNA. Virology. 1974 Aug;60(2):438–454. doi: 10.1016/0042-6822(74)90338-9. [DOI] [PubMed] [Google Scholar]
- Martin M. A., Howley P. M., Byrne J. C., Garon C. F. Characterization of supercoiled oligomeric SV40 DNA molecules in productively infected cells. Virology. 1976 May;71(1):28–40. doi: 10.1016/0042-6822(76)90091-x. [DOI] [PubMed] [Google Scholar]
- Mertz J. E., Berg P. Defective simian virus 40 genomes: isolation and growth of individual clones. Virology. 1974 Nov;62(1):112–124. doi: 10.1016/0042-6822(74)90307-9. [DOI] [PubMed] [Google Scholar]
- Mertz J. E., Berg P. Viable deletion mutants of simian virus 40: selective isolation by means of a restriction endonuclease from Hemophilus parainfluenzae. Proc Natl Acad Sci U S A. 1974 Dec;71(12):4879–4883. doi: 10.1073/pnas.71.12.4879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mertz J. E., Carbon J., Herzberg M., Davis R. W., Berg P. Isolation and characterization of individual clones of simian virus 40 mutants containing deletions duplications and insertions in their DNA. Cold Spring Harb Symp Quant Biol. 1975;39(Pt 1):69–84. doi: 10.1101/sqb.1974.039.01.012. [DOI] [PubMed] [Google Scholar]
- Rigby P. W., Dieckmann M., Rhodes C., Berg P. Labeling deoxyribonucleic acid to high specific activity in vitro by nick translation with DNA polymerase I. J Mol Biol. 1977 Jun 15;113(1):237–251. doi: 10.1016/0022-2836(77)90052-3. [DOI] [PubMed] [Google Scholar]
- Ritzi E., Levine A. J. Deoxyribonucleic acid replication in simian virus 40-infected cells. 3. Comparison of simian virus 40 lytic infection in three different monkey kidney cell lines. J Virol. 1970 Jun;5(6):686–692. doi: 10.1128/jvi.5.6.686-692.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roberts R. J., Breitmeyer J. B., Tabachnik N. F., Myers P. A. A second specific endonuclease from Haemophilus aegyptius. J Mol Biol. 1975 Jan 5;91(1):121–123. doi: 10.1016/0022-2836(75)90375-7. [DOI] [PubMed] [Google Scholar]
- Salzman N. P., Lebowitz J., Chen M., Sebring E., Garon C. F. Properties of replicating SV40 DNA molecules and mapping unpaired regions in SV40 DNA I. Cold Spring Harb Symp Quant Biol. 1975;39(Pt 1):209–218. doi: 10.1101/sqb.1974.039.01.027. [DOI] [PubMed] [Google Scholar]
- Sambrook J., Westphal H., Srinivasan P. R., Dulbecco R. The integrated state of viral DNA in SV40-transformed cells. Proc Natl Acad Sci U S A. 1968 Aug;60(4):1288–1295. doi: 10.1073/pnas.60.4.1288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharp P. A., Sugden B., Sambrook J. Detection of two restriction endonuclease activities in Haemophilus parainfluenzae using analytical agarose--ethidium bromide electrophoresis. Biochemistry. 1973 Jul 31;12(16):3055–3063. doi: 10.1021/bi00740a018. [DOI] [PubMed] [Google Scholar]
- Shenk T. E., Carbon J., Berg P. Construction and analysis of viable deletion mutants of simian virus 40. J Virol. 1976 May;18(2):664–671. doi: 10.1128/jvi.18.2.664-671.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Southern E. M. Detection of specific sequences among DNA fragments separated by gel electrophoresis. J Mol Biol. 1975 Nov 5;98(3):503–517. doi: 10.1016/s0022-2836(75)80083-0. [DOI] [PubMed] [Google Scholar]
- Syvanen M. In vitro genetic recombination of bacteriophage lambda. Proc Natl Acad Sci U S A. 1974 Jun;71(6):2496–2499. doi: 10.1073/pnas.71.6.2496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tegtmeyer P. Altered patterns of protein synthesis in infection by SV40 mutants. Cold Spring Harb Symp Quant Biol. 1975;39(Pt 1):9–15. doi: 10.1101/sqb.1974.039.01.004. [DOI] [PubMed] [Google Scholar]
- Vogt V. M. Purification and further properties of single-strand-specific nuclease from Aspergillus oryzae. Eur J Biochem. 1973 Feb 15;33(1):192–200. doi: 10.1111/j.1432-1033.1973.tb02669.x. [DOI] [PubMed] [Google Scholar]
- Waldeck W., Kammer K., Sauer G. Preferential integration of simian virus 40 deoxyribonucleic acid into a particular size class of CV-1 cell deoxyribonucleic acid. Virology. 1973 Aug;54(2):452–464. doi: 10.1016/0042-6822(73)90156-6. [DOI] [PubMed] [Google Scholar]
- Westphal H., Dulbecco R. Viral DNA in polyoma- and SV40-transformed cell lines. Proc Natl Acad Sci U S A. 1968 Apr;59(4):1158–1165. doi: 10.1073/pnas.59.4.1158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Westphal H. SV40 DNA strand selection by Escherichia coli RNA polymerase. J Mol Biol. 1970 Jun 14;50(2):407–420. doi: 10.1016/0022-2836(70)90201-9. [DOI] [PubMed] [Google Scholar]