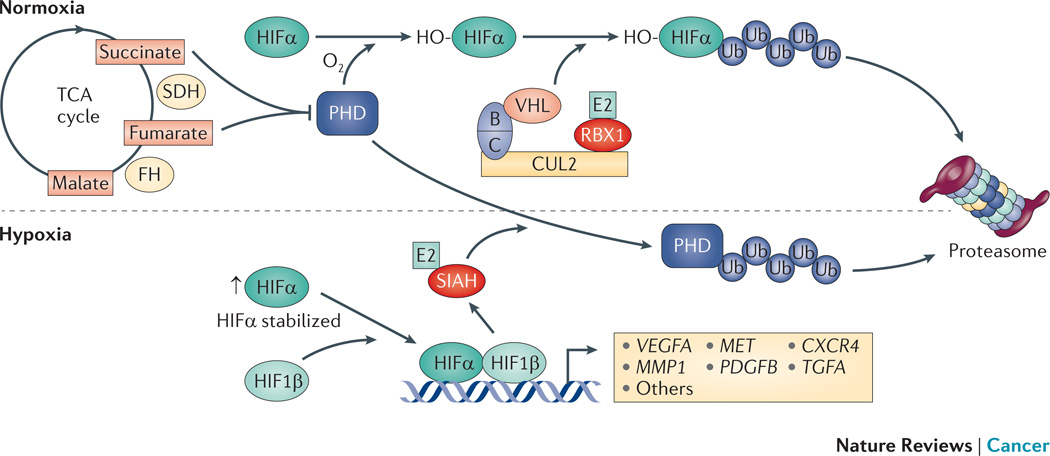
Figure 3. Hypoxia.
Hypoxia-inducible factor-α (HIFα) proteins are subunits of the HIF transcription factors, which regulate the expression of many genes associated with the response to hypoxia and proliferation. Under normoxic conditions the prolyl hydroxylase (PHD) proteins hydoxylate HIFα proteins, which creates a binding site recognized by the cullin RING ligase 2 (CRL2) – von Hippel Lindau tumor suppressor protein (VHL) E3 complex (CRL2VHL). This leads to proteasomal degradation of HIFα proteins. The PHD proteins are ubiquitylated and degraded by the seven in absentia homologue (SIAH) RING finger E3s that are themselves transcriptionally upregulated by HIF transcription factors in response to hypoxia152. The stability of HIFα proteins is also regulated by fumarate hydratase (FH) and succinate dehyrogenase (SDH), which are tumor suppressors associated with kidney cancer156. The loss of FH and SDH results in the accumulation of fumarate and succinate, respectively, which competitively inhibit PHD activity and prevent hydroxylation and VHL-mediated degradation of HIFα proteins leading to inappropriate upregulation of hypoxia inducible genes157. Figure reproduced with permission © Macmillan Publishers (2011).