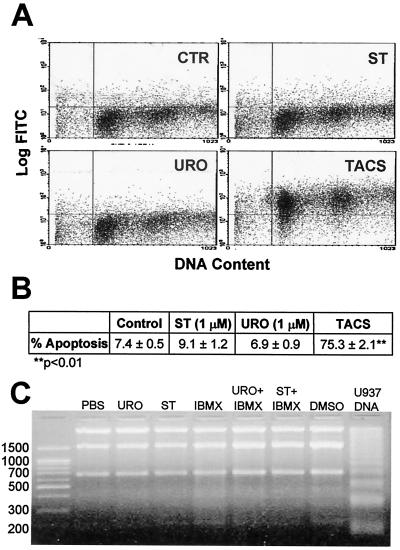
Figure 4.
(A) T84 cells were synchronized and stimulated to proliferate by l-glutamine, as described in Fig. 2A, with simultaneous addition of 1 μM ST, 1 μM uroguanylin (URO), or PBS (CTR). Incubations were continued for 24 h, and cells were trypsinized and pelleted, divided in 1 × 106 aliquots, fixed, and permeabilized by using Cytonin reagent. Biotinylated DNA was costained with both FITC-conjugated streptavidin and PI. The positive control (TACS) was generated by using the TACS-Nuclease provided with the FlowTACS kit. Flow cytometry analysis was performed as described in Materials and Methods, and data were plotted in two-dimensional format. Data are from a representative experiment. (B) Mean ± SEM of the percentage of FITC-positive T84 cells (apoptotic/necrotic) from three experiments performed as in A. (C) T84 cells (seeded at a density of 2 × 105 into 35-mm dishes) were cultured for 7 days in DMEM/F12, plus 10% FBS. Preconfluent monolayers were washed with DMEM (4.5 g/liter glucose, containing l-glutamine) and incubated in that media for 16 h. Cells were washed again in DMEM and then incubated for 2 h in that media supplemented as described in Materials and Methods (vehicle, PBS or DMSO; uroguanylin, URO). DNA was analyzed as described in Materials and Methods. The U937 positive control DNA was provided in the fragmentation analysis kit. Data are from a representative experiment.