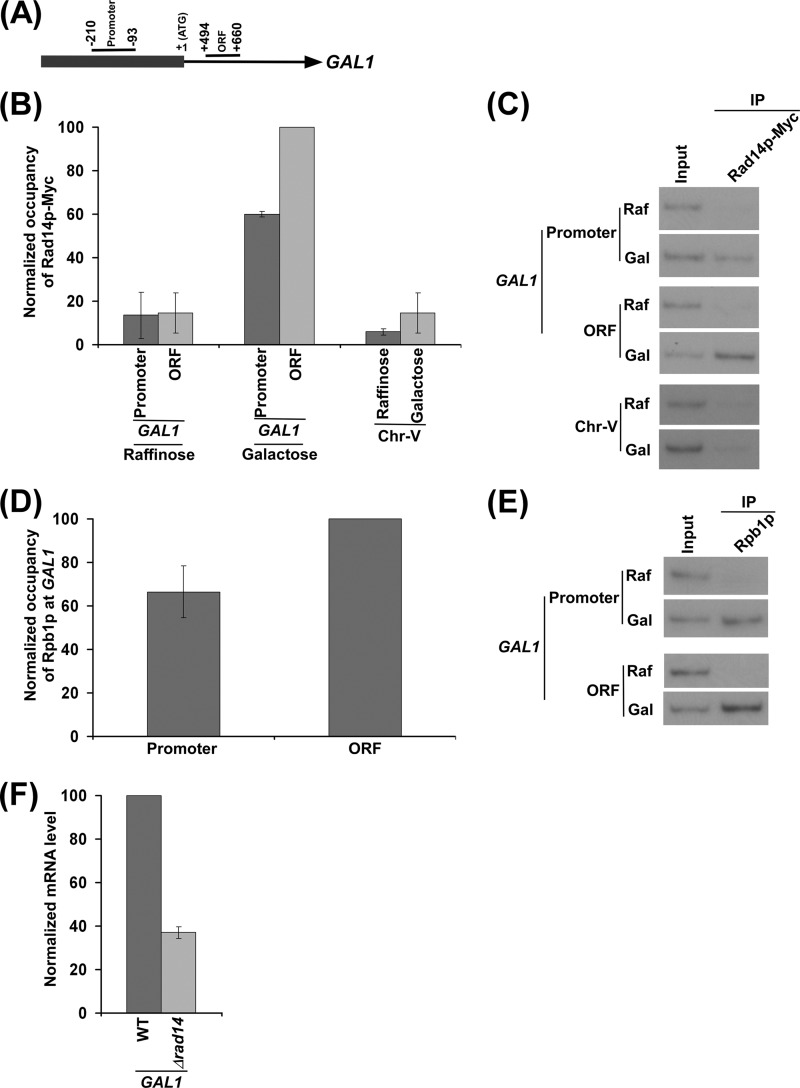
FIGURE 1.
Rad14p is associated with the promoter and coding sequence of the GAL1 gene in a transcription-dependent manner. A, a schematic diagram shows the PCR primer pairs located at the promoter and coding sequence (or ORF) of the GAL1 gene in the ChIP assay. The numbers are presented with respect to the position of the first nucleotide of the initiation codon (+1). B, Rad14p is associated with the promoter and coding sequence of the active GAL1 gene. The yeast strain expressing Myc epitope-tagged Rad14p (ZDY3) was grown in raffinose (YPR)- or galactose (YPG)-containing growth medium up to an A600 of 1.0 before cross-linking. Immunoprecipitation was performed using a mouse monoclonal antibody against the c-myc epitope-tag (9E10; Santa Cruz Biotechnology). Primer pairs targeted to the promoter and coding sequence of the GAL1 gene and an inactive region of the chromosome-V (Chr-V) were used for PCR analysis of the immunoprecipitated DNA samples. The maximum ChIP signal was set to 100, and other ChIP signals were normalized with respect to 100. The normalized ChIP signals (represented as normalized occupancy) were plotted in the form of a histogram. C, the autoradiograms for the data presented in the panel B are shown. IP, immunoprecipitate. D, RNA polymerase II is associated with the promoter and coding sequence of the active GAL1 gene. The yeast strain was grown in YPR up to an A600 of 0.9 and then switched to YPG for 90 min before cross-linking. Immunoprecipitation was performed using a mouse monoclonal antibody 8WG16 (Covance) against the C-terminal domain of the largest subunit (Rpb1p) of RNA polymerase II. The maximum ChIP signal was set to 100, and another ChIP signal was normalized with respect to 100. The normalized ChIP signal was plotted in the form of a histogram. E, the autoradiograms for the data presented in the panel D. F, RT-PCR analysis. Both the wild type and Δrad14 strains were grown as in panel D. The Δrad14 strain (PCY25) was generated in the W303a wild type background. GAL1 mRNA level in the wild type strain was set to 100, and the mRNA level in the Δrad14 strain was normalized with respect to 100. The normalized mRNA level was plotted in the form of a histogram.