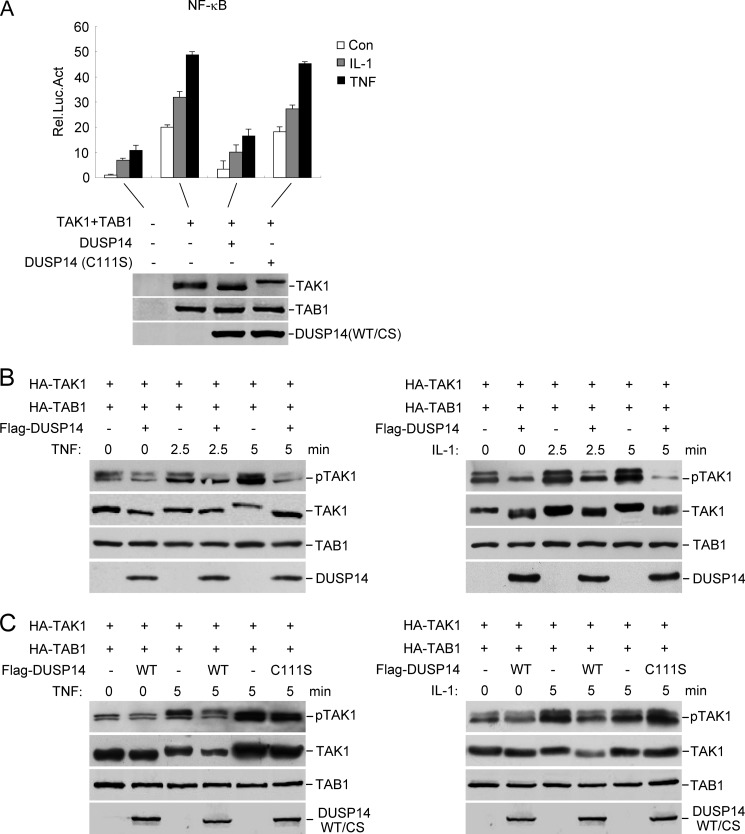
FIGURE 4.
DUSP14 dephosphorylates TAK1. A, the phosphatase activity of DUSP14 is required for its inhibitory effect on TAK1-mediated NF-κB activation. The 293 cells were transfected with the indicated plasmids, and NF-κB reporter assays were performed (upper panel). Expression of the transfected proteins was analyzed by immunoblotting with anti-FLAG and anti-HA antibodies (lower panel). The graph shows means ± S.D. (n = 3). Rel. Luc. Act., relative luciferase activity; Con, control; CS, DUSP14(C111S). B, DUSP14 dephosphorylates TAK1 at Thr-187. The 293 cells (4 × 105) were transfected with the indicated expression plasmids. Twenty-four hours after transfection, cells were left untreated or treated with TNF (10 ng/ml) or IL-1 (10 ng/ml) for the indicated times. Cell lysates were analyzed by immunoblotting with anti-phospho-TAK1 (Thr-187), anti-HA, and anti-FLAG antibodies. C, the phosphatase activity of DUSP14 is required for its ability to dephosphorylate TAK1 at Thr-187. The experiments were performed as described for B.