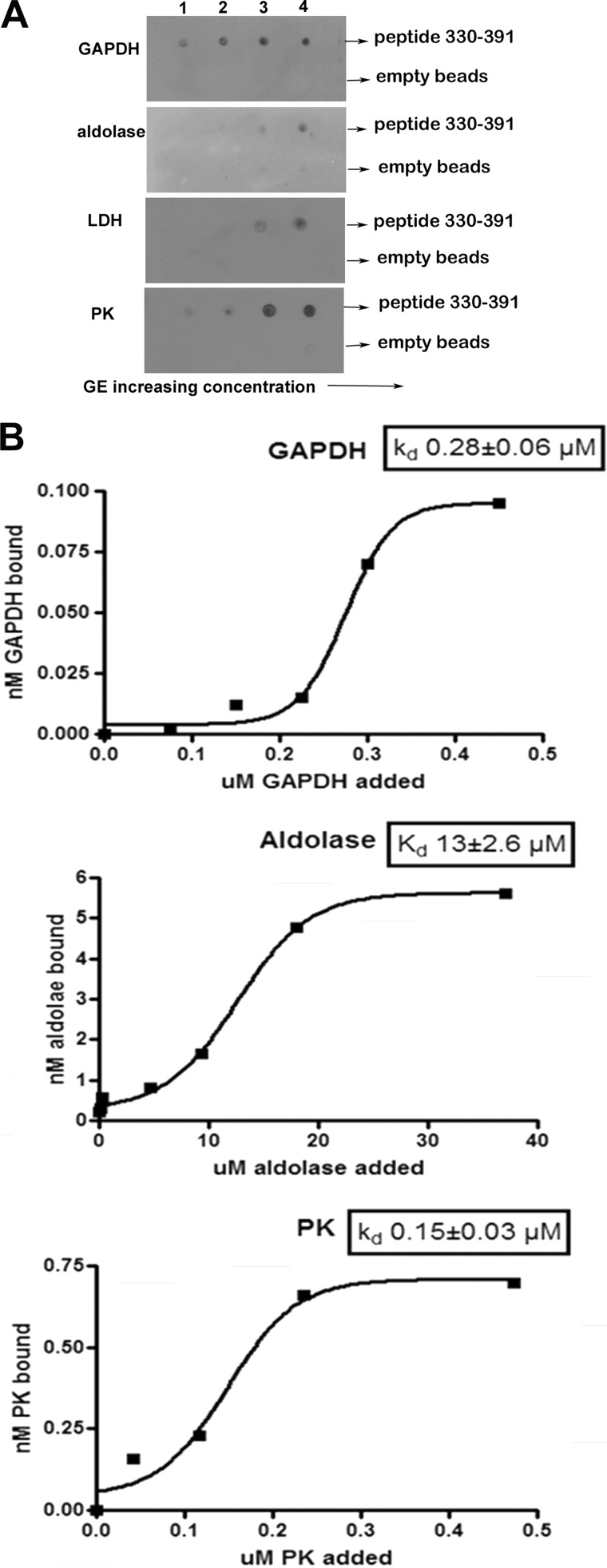
FIGURE 6.
Effect of increasing GE concentration on the binding of GEs to the peptide comprising residues 330–391 of band 3. A, evaluation of the affinity of GEs for the His-tagged fusion protein containing residues 330–391 of band 3 by nickel bead pull-down assay. Fusion protein (0.17 μmol) was incubated with increasing amounts (5–50 nmol) of GE in a total volume of 100 μl overnight at 4 °C. Nickel beads blocked with binding buffer containing 5% BSA were added, and the His-tagged fragment with bound GE was collected and washed before analysis. A similar sample containing only GE was run in parallel and dot-blotted as a control for nonspecific binding to the nickel beads. The concentrations of GE tetramers applied to the blot in each lane were as follows. Lane 1, GAPDH, 54 nm; aldolase, 51 nm; LDH, 50 nm; PK, 51 nm. Lane 2, GAPDH, 134 nm; aldolase, 126 nm; LDH, 126 nm; PK, 123 nm. Lane 3, GAPDH, 268 nm; aldolase, 252 nm; LDH, 246 nm; PK, 249 nm. Lane 4, GAPDH, 536 nm; aldolase, 504 nm; LDH, 492 nm; PK, 497 nm. B, determination of the Kd of each GE for the peptide 330–391 of band 3. Data are plotted using GraphPad Prism 4 and fitted to a Boltzman sigmoidal equation.