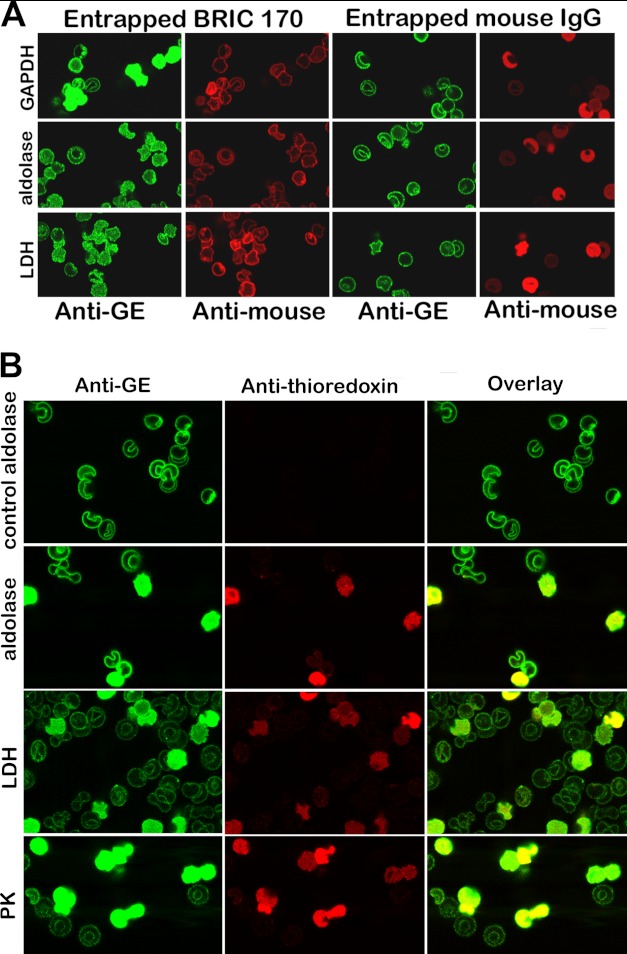
FIGURE 8.
Displacement of GEs from erythrocyte membranes by entrapment of a monoclonal antibody to residues 368–382 of band 3 (BRIC 170) (A) or a fusion protein comprising residues 330–391 linked to thioredoxin (B). A, the first and second columns show the location of endogenous GEs (green stain) and the entrapment of BRIC 170 (red stain). Parallel control experiments following entrapment of a nonspecific mouse IgG are shown in columns 3 and 4. Quantitation of the intensity of GE staining in the cytosol using an Olympus Fluo View microscope version 2.1 reveals that the cytosolic GE staining for GAPDH, aldolase, and LDH is 20, 4, and 8 times greater on average in BRIC 170-entrapped cells than in isotype control-entrapped cells. B, a fusion protein containing peptide 330–391 linked to thioredoxin was entrapped into resealed ghosts, and its effect on GE binding was evaluated by confocal microscopy. A control in which no peptide fusion protein was entrapped during the incubation step with leaky ghosts was run in parallel and is shown in the first row (labeled control aldolase). The first column shows the staining of the GEs; the second column identifies the “ghosts” that have successfully entrapped the fusion protein (red stain of the anti-thioredoxin antibody); and the third column shows the overlay of both stains.