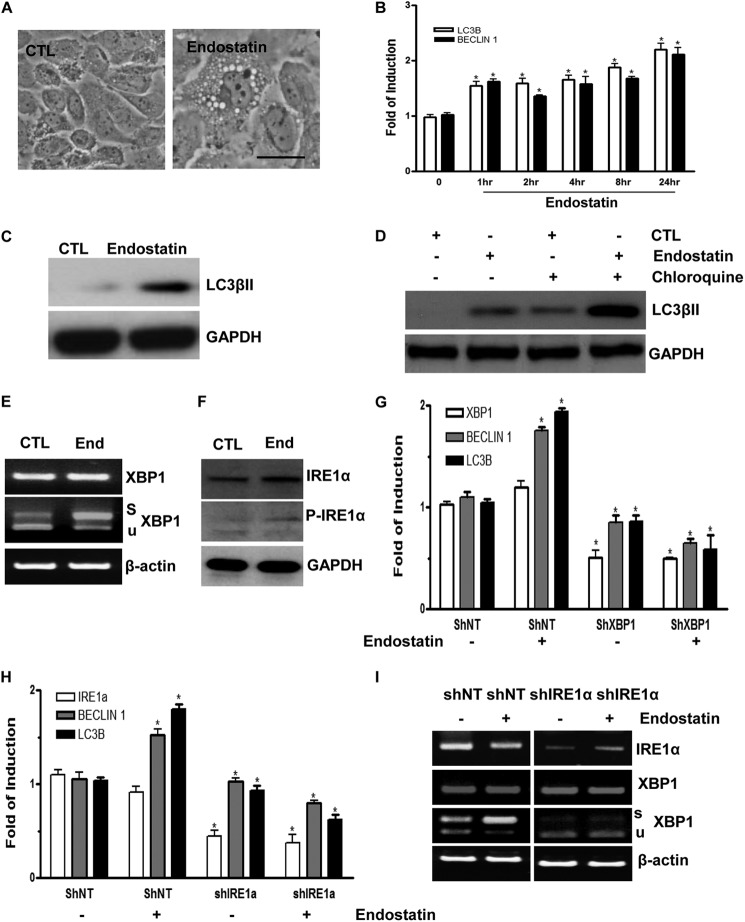
FIGURE 5.
Endostatin induces autophagy in ECs through IRE1α-mediated XBP1 mRNA splicing. A, endostatin-induced autophagy in ECs. Images were taken at 24 h after 20 ng/ml endostatin treatment. Scale bar, 25 μm. B, endostatin up-regulates autophagic gene expression at the mRNA level in time course experiments. C, ECs stimulated with endostatin for 24 h showing induced LC3βII expression at the protein level. D, ECs were treated with endostatin for 24 h prior to chloroquine treatment for 6 h, and Western blot for LC3βII is shown. E, endostatin-induced XBP1 mRNA splicing and IRE1α phosphorylation. ECs were treated with endostatin for 4 h, followed by RT-PCR plus PstI digestion and Western blot analysis (F). Note that endostatin induced IRE1α phosphorylation and robust XBP1 mRNA splicing. CTL, control; End, endostatin; s, spliced band; u, unspliced band. β-Actin and GAPDH were included as mRNA and protein loading control, respectively. G, knockdown of XBP1 abolished endostatin-induced autophagy gene expression. Non-target (shNT) or XBP1 (shXBP1) shRNA lentiviruses-infected ECs (for 72 h) were treated with endostatin for 4 h, followed by real-time PCR analysis of autophagy gene expression. H and I, knockdown of IRE1α abolished endostatin-induced autophagy gene expression. Non-target (shNT) or IRE1α (shIRE1α) shRNA lentivirus-infected ECs (for 72 h) were treated with endostatin for 4 h, followed by real-time PCR analysis of autophagy gene expression or routine RT-PCR. Note that treatment with endostatin even decreased autophagy gene expression in IRE1α-deficient cells. *, p < 0.05. Data presented are representative of or an average of three independent experiments. Error bars, S.E.