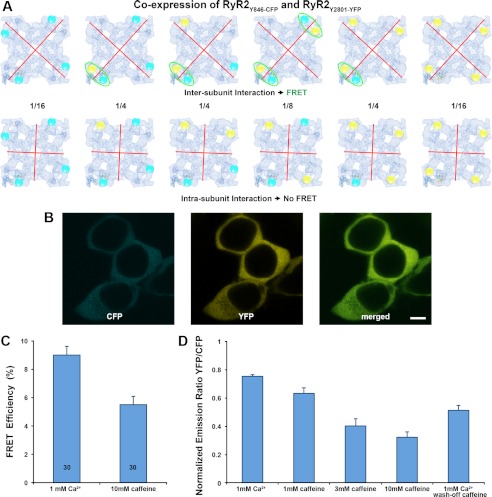
FIGURE 7.
FRET analysis in HEK293 cells that co-express RyR2Y846-CFP and RyR2Y2801-YFP. A, models of six possible hybrid RyR2 tetramer molecules when two cDNAs that encode RyR2Y846-CFP and RyR2Y2801-YFP are co-expressed. The cyan and yellow spheres represent the CFP and YFP. Red lines represent the possible boundary between RyR subunits. The top row shows six RyR2 tetramer structures for the situation in which two structural domains bearing Tyr-846-CFP and Tyr-2801-YFP belong to two different subunits (i.e. an intersubunit interaction). In this case, four out of six structures have at least one FRET pair within one corner of the RyR cytoplasmic assembly (highlighted by green ellipses). The bottom row shows six RyR2 tetramer structures for the case in which the two structural domains bearing Tyr-846-CFP and Tyr-2801-YFP are contained within one RyR subunit (i.e. an intrasubunit interaction). In this case, no FRET signal will be detected, because the distance between CFP and YFP in two separate corners is over 200 Å. Numbers between two rows are the mathematical probability of a ratio among six possible structures when RyR2 tetramers are formed randomly. B, images of live HEK293 cells co-transfected with cDNAs of RyR2Y846-CFP and RyR2Y2801-YFP showing co-localization of CFP and YFP; scale bar, 5 μm. C, FRET efficiency determined by photo-bleaching of acceptor. Data are mean ± S.E., with the number of cells indicated in the bars. D, change of FRET signal determined by monitoring acceptor/donor emission ratio. Data are mean ± S.E., averaged from seven separate experiments.