Figure 4.
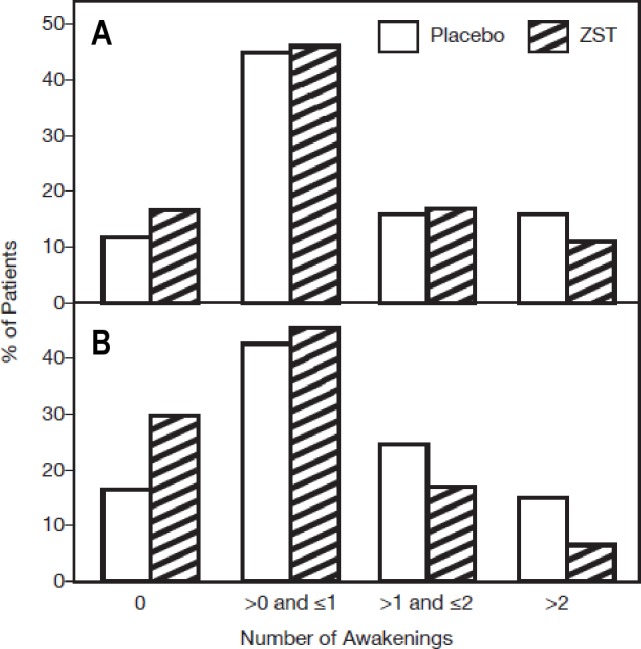
NAWMOTN during the run-in and double-blind treatment period. Continuous values obtained by averaging were then categorized as shown. The row mean score statistic was employed for categorized mean NAWMOTN. (A) Baseline NAWMOTN is the average of values collected during the 2-wk placebo single-blind screening period for those nights study medication was taken. No statistical difference between groups. (B) Four-wk treatment average. P < 0.001. NAWMOTN, subjective number of awakenings following middle-of-the-night awakening; ZST, 3.5-mg zolpidem tartrate sublingual tablet.
