Abstract
Background
The mechanisms contributing to the pain of migraine are poorly understood although activation of afferent nociceptors in the trigeminovascular system has been proposed as a key event. Prior studies have shown that dural-afferent nociceptors are sensitive to both osmotic and mechanical stimuli. Based on the sensitivity to these stimuli we hypothesized that dural afferents express the osmo/mechano-sensitive channel transient receptor-potential vanilloid 4 (TRPV4).
Methods
These studies used in vitro patch-clamp electrophysiology of trigeminal neurons retrogradely labeled from the dura to examine the functional expression of TRPV4. Additionally, we used a rat headache model in which facial/hind paw allodynia following dural stimulation is measured to determine whether activation of meningeal TRPV4 produces responses consistent with migraine.
Results
These studies found that 56% and 49% of identified dural afferents generate currents in response to hypotonic solutions and 4α-PDD, respectively. The response to these stimuli indicates that dural afferents express TRPV4. Activation of meningeal TPRV4 using hypotonic solution or 4α-PDD in vivo resulted in both facial and hind paw allodynia that was blocked by the TRPV4 antagonist RN1734.
Conclusion
These data indicate that activation of TRPV4 within the meninges produces afferent nociceptive signaling from the head that may contribute to migraine headache.
Keywords: Pain, migraine, TRPV4, headache, meninges
Introduction
Migraine headache is one of the most common chronic pain disorders, affecting up to 33% of women and 13% of men at some point in their lifetime (1). Despite its prevalence, the mechanisms contributing to migraine headache are still poorly understood. One likely source of headache is activation of afferent signaling from the cranial meninges. Earlier work in preclinical models has found that trigeminal pain-sensing neurons (nociceptors) innervating the dura are sensitive to mechanical stimulation (2–4). This may explain the worsening of migraine headache in response to increased mechanical forces such as sudden head movements or increased intracranial pressure during coughing. Strassman and colleagues also showed that trigeminal afferent nociceptors can be activated by dural application of solutions with either increased or decreased osmolarity (4). These findings suggest that dural afferents express the osmo/mechano-sensitive ion channel transient receptor-potential vanilloid 4 (TRPV4).
First described as a hypo-osmolar-activated ion channel, TRPV4 is a non-selective Ca2+ channel that responds to warm temperature as well as mechanical stimulation. Expression of mRNA for TRPV4 is found in the trigeminal ganglion (5) and functional effects of TRPV4 activation can be measured in trigeminal neurons in vitro (6,7). TRPV4 can be activated by changes in osmolarity as well as by mechanical stimuli (8,9) and mice lacking TRPV4 exhibited a loss in both osmotic and pressure sensation (10,11). Thus, TRPV4 is a possible candidate that contributes to the osmo- and mechano-sensitivity of dural afferent nociceptors. Using in vitro electrophysiology of identified dural afferents and a rat behavioral model of migraine, the purpose of the present studies was to determine whether dural afferents express TRPV4 and whether activation of this channel contributes to migraine-related pain behavior.
Materials and methods
Animals
Adult, male Sprague Dawley rats (150–175 g for patch clamp, 250–300 g for behavior) were maintained in a climate-controlled room on a 12-hour light/dark cycle with food and water ad libitum. All procedures were performed in accordance with the policies and recommendations of the International Association for the Study of Pain, the National Institutes of Health guidelines for handling and use of laboratory animals, and by the Institutional Animal Care and Use Committee of the University of Arizona.
Surgery
Tracer injection
Dural afferents were identified as previously described (12). Briefly, 7 days prior to the sacrifice, animals were anesthetized. Under a dissecting microscope, two holes (3 mm in diameter) were made in the skull using a Dremel Multipro 395 fitted with a dental drill bit (Stoelting) leaving a thin layer of bone at the bottom of the hole. Fine forceps were used to carefully remove the remaining bone and expose but not damage the dura. Five (5) μl Fluoro-Gold (4% in SIF: synthetic-interstitial fluid) was then applied onto the dura. A small piece of gel foam was retained in the hole to increase absorption of dye and prevent dye spread out of the holes. The holes were covered with bone wax to prevent tracer leakage. Immediately postoperatively, animals received a single subcutaneous injection of gentamicin (8 mg/kg) to minimize infection. Only animals with intact dura were used for further experiments.
Dura cannulation
Dura cannulae were implanted as previously described (12). Animals were anesthetized with a combination of ketamine and xylazine (80 mg/kg and 12 mg/kg; Sigma-Aldrich). A 2 cm incision was made to expose the skull. A 1 mm hole (above the transverse sinus; 2 mm left of sagittal suture and 2 mm anterior to lambdoid suture) was made with a hand drill (Plastics One) to carefully expose the dura. A guide cannula (Plastics One), designed to extend 0.5 mm from the pedestal to avoid irritation of the dural tissue, was inserted into the hole and sealed into place with glue. Two additional 1 mm holes were made rostrally to the cannula to receive stainless steel screws (Small Parts), and dental acrylic was used to fix the cannula to the screws. A dummy cannula (Plastics One) was inserted to ensure patency of the guide cannula. Immediately post-operatively, animals received a single subcutaneous injection of gentamicin (8 mg/kg) to minimize infection. Rats were housed separately and allowed 6–8 days of recovery.
Cell culture
Seven days following Fluoro-Gold application, trigeminal ganglia (TG) were removed, enzymatically treated, and mechanically dissociated as previously described (12). Rats were anesthetized with isoflurane (Phoenix Pharmaceuticals) and sacrificed by decapitation. The TG were removed and placed in ice-cold Hanks balanced-salt solution (divalent free). Ganglia were cut into small pieces and incubated for 25 minutes in 20 U/ml Papain (Worthington) followed by 25 minutes in 3 mg/ml Collagenase Type II (Worthington). Ganglia were then triturated through fire-polished Pasteur pipettes and plated on poly-D-lysine (Becton Dickinson) and laminin (Sigma)-coated plates. After several hours at room temperature to allow adhesion, cells were cultured in a room temperature, humidified chamber in Liebovitz L-15 medium supplemented with 10% FBS, 10 mM glucose, 10 mM HEPES and 50 U/ml penicillin/streptomycin. Cells were used within 24 hours of plating.
Electrophysiology
Whole cell patch-clamp experiments were performed on isolated rat TG using a MultiClamp 700B (Axon Instruments) patch-clamp amplifier and pClamp 10 acquisition software (Axon Instruments). Recordings were sampled at 5 kHz and filtered at 1 kHz (Digidata 1322A, Axon Instruments). Pipettes (OD: 1.5 mm, ID: 0.86 mm, Sutter Instrument) were pulled using a P-97 puller (Sutter Instrument) and heat polished to 2.5–4 MΩ resistance using a microforge (MF-83, Narishige). Series resistance was typically <7 MΩ and was compensated 60%. All recordings were performed at room temperature. A Nikon TE2000-S microscope equipped with a mercury arc lamp (X-Cite® 120) was used to identify FG-labeled dural afferents. Data were analyzed using Clampfit 10 (Molecular Devices) and Origin 8 (OriginLab). Pipette solution contained (in mM) 140 KCl, 11 EGTA, 2 MgCl2, 10 NaCl, 10 HEPES, 2 MgATP, and 0.3 Na2GTP, 1 CaCl2, pH 7.3 (adjusted with N-methyl glucamine), and was ~315 mOsm. External solution contained (in mM) 135 NaCl, 2 CaCl2, 1 MgCl2, 5 KCl, 10 glucose, 10 HEPES, pH 7.4 (adjusted with N-methyl glucamine), and was ~300 mOsm. Hypotonic solution contained (in mM) 88 NaCl, 2 CaCl2, 1 MgCl2, 5 KCl, 10 HEPES, pH 7.4 (adjusted with N-methyl glucamine), and was ~200 mOsm or was ~260 mOsm following the addition of 60 D-mannitol. Isotonic solution contained (in mM) 88 NaCl, 2 CaCl2, 1 MgCl2, 5 KCl, 106 D-mannitol, 10 HEPES, pH 7.4 (adjusted with N-methyl glucamine), and was 300 mOsm. Isotonic solution (with equimolar Na+ concentration to the hypotonic solution) was applied to obtain a stable baseline before changing to hypotonic solution. Solutions were rapidly changed during recordings using gravity-fed flow pipes positioned near the cell and controlled by computer-driven solenoid valves (Automate Scientific, ValveLink 8.2). The solution exchange time was ~20 ms. The application time of TRPV4 activators is 1 minute. A cut-off of 50 pA was selected as minimum amplitude for response for both the TRPV4 activators.
Behavior testing
Cutaneous allodynia measurements were performed as previously described (12,13). Rats were acclimated to suspended Plexiglas chambers (30 cm long ×15 cm wide ×20 cm high) with a wire mesh bottom (1 cm2). Ten (10) μl of vehicle or testing solution was injected through an injection cannula (Plastics One) cut to fit the guide cannula. Withdrawal thresholds to probing the face and hind paws were determined at 1-hour intervals after administration. A behavioral response to calibrated von Frey filaments applied to the midline of the forehead, at the level of the eyes, was indicated by a sharp withdrawal of the head. Paw withdrawal (PW) thresholds were determined by applying von Frey filaments to the plantar aspect of the hind paws, and a response was indicated by a withdrawal of the paw. The withdrawal thresholds were determined by the Dixon up–down method. Maximum filament strengths were 8 and 15 gm for the face and hind paws, respectively.
Data analysis
All data are presented as means ± SEM unless otherwise noted. Behavioral studies among groups and across time were analyzed by two-factor ANOVA. Data were converted to area over the time-effect curve.
Solutions
Fluoro-Gold was purchased from Fluorochrome, LLC, and dissolved in synthetic interstitial fluid (SIF: pH 7.4, 310 mOsm) to 4%. 4α-PDD (4a-phorbol 12,13-didecanote, a selective TRPV4 agonist) was purchased from Sigma; it was dissolved in DMSO to 10 mM as a stock solution and diluted to 2 μM. RN1734 was purchased from Tocris (14). For behavior experiments, stock 4α-PDD (10 mM in DMSO) and stock RN1734 (10 mM in DMSO) were diluted to final concentrations of 100 μM and 500 μM, respectively in pH 7.4 SIF. Hypotonic solution was made by diluting SIF 1 to 10 and adjusting the pH to 7.4.
Results
TRPV4 activators evoke currents in identified dural afferents
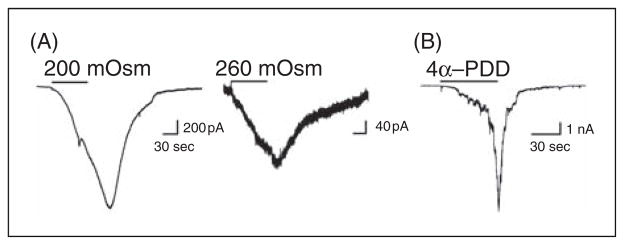
Patch clamp electrophysiology was performed on TG neurons in cultures from rats in which Fluoro-Gold was previously applied onto the dura. Retrogradely-labeled cells were selected for recording as these cells represent dural-projecting neurons. Examples of currents evoked by a 1 minute application of TPRV4 activators from a representative dural afferent are shown in Figure 1A and 1B. Among 87 dural afferents from 13 rats, 56% exhibit TRPV4-like currents in response to exposure to hypotonic solutions of 200 and 260 mOsm, and 49% exhibited TRPV4-like 4α-PDD evoked currents.
Figure 1.
Dural afferents generate TRPV4-like currents in response to hypotonic stimuli and 4α-PDD. (A) Currents from the same representative dural afferent neuron in response to a 1 minute application of 200 mOsm or 260 mOsm solutions. Currents in response to hypotonic stimuli were observed in 56% of dural afferents. (B) Currents in response to a 1 minute application of 4α-PDD in a representative dural afferent. 4α-PDD-induced currents were observed in 49% of dural afferents.
Cutaneous allodynia following activation of TRPV4 within the dura
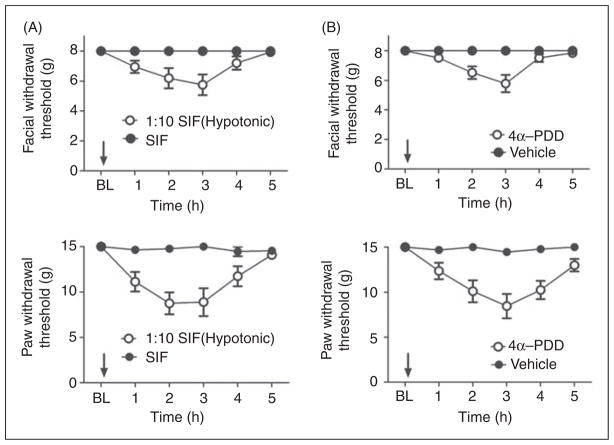
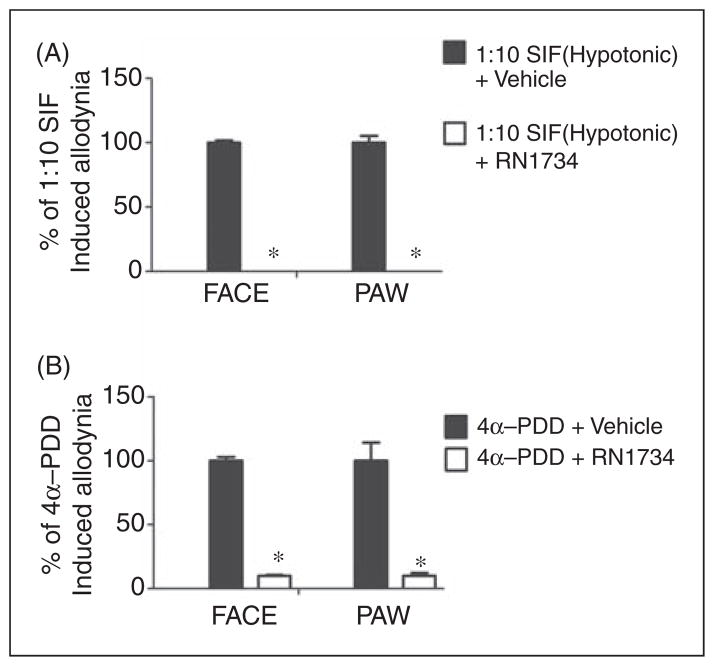
Application of hypotonic solution (SIF 1:10) to the dura produced significant time-dependent and reversible reductions in withdrawal thresholds to tactile stimuli applied to the face or the hind paws (Figure 2A). Maximal effects occurred 2 hours after hypotonic solution application and sensory thresholds returned to baseline by 5 hours. Similarly, application of 4α-PDD to the dura produced significant time-dependent and reversible facial and hind paw allodynia with the same time course as that observed following hypotonic solution (Figure 2B). In order to determine whether hypotonic solution and 4α-PDD are acting via TRPV4, the TRPV4 antagonist RN1734 was co-applied with the activating stimulus onto the dura. This antagonist has been tested in vitro previously against a panel of TRP channels and found to be a selective antagonist for TRPV4 (14). Importantly, this antagonist was also shown to block TRPV4 activation by both hypotonic stimuli as well as 4α-PDD. Co-application of the TRPV4 antagonist RN1734 with hypotonic SIF blocked the hypotonicity-induced allodynia (Figure 3A). Co-application of RN1734 with 4α-PDD also blocked the 4α-PDD induced allodynia (Figure 3B). Importantly, RN1734 application alone did not produce behavioral responses that were different than vehicle treatment (data not shown). Additionally, co-application of RN1734 and capsaicin did not differ from a capsaicin plus vehicle administration in producing allodynia, indicating that this antagonist is selective for TRPV4 in vivo and does not block all sensory input from the dura (Supplementary Figure 1). These behavioral data indicate that activation of TRPV4 within the dura produces afferent nociceptive signaling and a migraine-related behavioral response.
Figure 2.
Application of hypotonic solution and 4α-PDD to the dura elicited cutaneous allodynia via activation of TRPV4. (A) Withdrawal thresholds to tactile stimuli applied to the face (top) and the hind paws (bottom) were measured in rats before and for 5 hours after dural application of 10% SIF (n =15) or normal osmolarity SIF (n =11). For both facial and hind paw responses, two-factor analysis of variance with repeated measurement indicated that response thresholds of hypotonic solution-treated rats were significantly (p <0.0001) less than those of standard SIF-treated rats. (B) Withdrawal thresholds to tactile stimuli applied to the face (top) and the hind paws (bottom) were measured in rats before and for 5 hours after dural application of 4α-PDD (100 μM) (n =16) or vehicle (n =16). For both facial and hind paw responses, two-factor analysis of variance with repeated measurement indicated that response thresholds of 4α-PDD-treated rats were significantly (p <0.0001) less than those of vehicle treated rats.
Figure 3.
Cutaneous allodynia produced by hypotonic stimuli or 4α-PDD is blocked by a TRPV4 antagonist. Application of 10% SIF or 4α-PDD was given either alone or in the presence of RN1734 (500 μM, n =16 and 8 for 10% SIF and 4α-PDD, respectively). Vehicle control was SIF containing 5% DMSO which produced no allodynia. Significant (p <0.05) differences among means for each group were determined by analysis of variance followed by Dunnett’s post hoc test. Co-application of RN1734 significantly abolished behavioral signs of tactile allodynia of the face and hind paw (p <0.05). RN1734 alone did not induce allodynia.
Discussion
Although the mechanisms contributing to migraine are poorly understood, it is likely that migraine pain is a result of activation of nociceptive signaling from the meninges. Uncovering the receptors and proteins that lead to activation of dural afferents will not only contribute to the understanding of migraine headache pathophysiology, it may also propose new targets for treatment of migraine pain.
The results of the present study implicate TRPV4 in the mechanisms contributing to migraine headache. Electrophysiological recordings indicated that approximately half of the dural afferents studied express TRPV4 as they generated currents in response to 4α-PDD and hypotonic solutions. Further, activation of TRPV4 within the dura of freely moving animals induced migraine-like behaviors (i.e. cephalic and extracephalic allodynia) that were blocked by an antagonist of the TRPV4 channel. Thus, activation of dural afferent TRPV4 is one possible mechanism contributing to the pathophysiology of migraine headache and this finding suggests blockers of TRPV4 as novel therapeutics.
While these studies demonstrate that activation of TRPV4 within the meninges produces dural afferent-activation and migraine-related behavior, they do not identify the endogenous mechanism of TRPV4 activation. Hypotonic stimuli were used throughout the manuscript as an activator of TRPV4 but there is currently no evidence that plasma osmolarity decreases before or during migraine, particularly to the extent used here (i.e. 260 mOsm and below). Thus, it is unclear whether decreased osmolarity is a mechanism leading to migraine.
The TRPV4 channel may be activated/sensitized downstream of other receptors. A recent study found sensitization of threshold mechanical responses of dural afferents in vivo following activation of the protease-activated receptor 2 (PAR2) (15). PAR2 is activated by its N-terminus, which is cleaved by extracellular proteases including tryptase. One likely source of these proteases (in addition to other pro-inflammatory mediators) is mast cells, which have been previously implicated in migraine pathophysiology (16–18). Consistent with this idea is a prior study indicating that PAR2 agonists sensitize TRPV4 currents in DRG neurons and lead to mechanical hyperalgesia in the hind paw and hypersensitivity to colorectal distension that is dependent on expression of this channel (19–21). Alternatively, TPRV4 is activated by arachidonic acid P450 epoxygenase-dependent metabolites such as epoxyeicosatrienoic acids (22) so endogenous lipid mediators may contribute to channel activation.
Although not directly tested here, it is tempting to speculate that TRPV4 contributes to the mechanosensitivity of dural afferents and may play a role in the worsening of headache during cough or changes in intracranial pressure. Additionally, the throbbing pain of migraine has been proposed to come from pulsatile flow of blood and distention of cranial arteries during systole. Vessel stretch could activate TRPV4-expressing nerve endings that are in close proximity to blood vessels under pathological conditions such as those that sensitize TRPV4. It should be noted however that a previous study has shown a lack of correlation between the subjective experience of throbbing pain and the arterial pulse (23), so the role of the vasculature in the throbbing nature of migraine is still not clear. Nonetheless, mechanical stimulation is known to activate TRPV4 (8,9) and defects in pressure sensation were observed in mice lacking TRPV4 (10,11). Administration of inflammatory soup (IS) into the paw results in hypersensitivity to osmotic and mechanical stimulation that is present in wild-type but not TRPV4-null mice. These observations suggest TRPV4 can be sensitized by pro-inflammatory mediators including those found in experimental IS and its mechanosensitivity is enhanced under inflammatory conditions. This effect is decreased following antisense-mediated knockdown of TRPV4 expression and is not observed in TRPV4 knockout mice (24). Together with the present findings, these studies suggest that TRPV4 may contribute to the mechanosensitivity of dural afferents but more detailed studies are required before this conclusion can be more definitively drawn.
Although treatments for migraine headache are currently available, these treatments lack adequate efficacy for many patients. Development of new therapeutics has been a slow process due, in part, to a lack of new drug targets. The results of the present study suggest that TRPV4 may be a novel target for the treatment of migraine headache. Future studies will determine whether targeted therapies blocking this channel will bring relief to the large numbers of migraine patients that are currently left without adequate treatment.
Supplementary Material
Acknowledgments
Funding
This work was supported by funding from the University of Arizona Foundation (GD), the American Pain Society (GD), the National Headache Foundation (GD), and the National Institutes of Health (R01NS072204, GD).
Footnotes
Conflicts of interest
The authors declare that they have no conflicts of interest.
Reprints and permissions: sagepub.co.uk/journalsPermissions.nav
References
- 1.Launer LJ, Terwindt GM, Ferrari MD. The prevalence and characteristics of migraine in a population-based cohort: the GEM study. Neurology. 1999;53:537–542. doi: 10.1212/wnl.53.3.537. [DOI] [PubMed] [Google Scholar]
- 2.Kaube H, Hoskin KL, Goadsby PJ. Activation of the trigeminovascular system by mechanical distension of the superior sagittal sinus in the cat. Cephalalgia. 1992;12:133–136. doi: 10.1046/j.1468-2982.1992.1203133.x. [DOI] [PubMed] [Google Scholar]
- 3.Levy D, Strassman AM. Mechanical response properties of A and C primary afferent neurons innervating the rat intracranial dura. J Neurophysiol. 2002;88:3021–3031. doi: 10.1152/jn.00029.2002. [DOI] [PubMed] [Google Scholar]
- 4.Strassman AM, Raymond SA, Burstein R. Sensitization of meningeal sensory neurons and the origin of headaches. Nature. 1996;384:560–564. doi: 10.1038/384560a0. [DOI] [PubMed] [Google Scholar]
- 5.Kitahara T, Li HS, Balaban CD. Changes in transient receptor potential cation channel superfamily V (TRPV) mRNA expression in the mouse inner ear ganglia after kanamycin challenge. Hear Res. 2005;201:132–144. doi: 10.1016/j.heares.2004.09.007. [DOI] [PubMed] [Google Scholar]
- 6.Chen L, Liu C, Liu L. The modulation of voltage-gated potassium channels by anisotonicity in trigeminal ganglion neurons. Neuroscience. 2008;154:482–495. doi: 10.1016/j.neuroscience.2008.03.046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Chen L, Liu C, Liu L. Changes in osmolality modulate voltage-gated calcium channels in trigeminal ganglion neurons. Brain Res. 2008;1208:56–66. doi: 10.1016/j.brainres.2008.02.048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Liedtke W, Choe Y, Marti-Renom MA, et al. Vanilloid receptor-related osmotically activated channel (VR-OAC), a candidate vertebrate osmoreceptor. Cell. 2000;103:525–535. doi: 10.1016/s0092-8674(00)00143-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Liedtke W, Tobin DM, Bargmann CI, et al. Mammalian TRPV4 (VR-OAC) directs behavioral responses to osmotic and mechanical stimuli in Caenorhabditis elegans. Proc Natl Acad Sci U S A. 2003;100(Suppl 2):14531–14536. doi: 10.1073/pnas.2235619100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Mizuno A, Matsumoto N, Imai M, et al. Impaired osmotic sensation in mice lacking TRPV4. Am J Physiol Cell Physiol. 2003;285:C96–C101. doi: 10.1152/ajpcell.00559.2002. [DOI] [PubMed] [Google Scholar]
- 11.Suzuki M, Mizuno A, Kodaira K, et al. Impaired pressure sensation in mice lacking TRPV4. J Biol Chem. 2003;278:22664–22668. doi: 10.1074/jbc.M302561200. [DOI] [PubMed] [Google Scholar]
- 12.Yan J, Edelmayer RM, Wei X, et al. Dural afferents express acid-sensing ion channels: a role for decreased meningeal pH in migraine headache. Pain. 2011;152:106–113. doi: 10.1016/j.pain.2010.09.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Edelmayer RM, Vanderah TW, Majuta L, et al. Medullary pain facilitating neurons mediate allodynia in headache-related pain. Ann Neurol. 2009;65:184–193. doi: 10.1002/ana.21537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Vincent F, Acevedo A, Nguyen MT, et al. Identification and characterization of novel TRPV4 modulators. Biochem Biophys Res Commun. 2009;389:490–494. doi: 10.1016/j.bbrc.2009.09.007. [DOI] [PubMed] [Google Scholar]
- 15.Zhang XC, Levy D. Modulation of meningeal nociceptors mechanosensitivity by peripheral proteinase-activated receptor-2: the role of mast cells. Cephalalgia. 2008;28:276–284. doi: 10.1111/j.1468-2982.2007.01523.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Zhang XC, Strassman AM, Burstein R, et al. Sensitization and activation of intracranial meningeal nociceptors by mast cell mediators. J Pharmacol Exp Ther. 2007;322:806–812. doi: 10.1124/jpet.107.123745. [DOI] [PubMed] [Google Scholar]
- 17.Levy D, Burstein R, Kainz V, et al. Mast cell degranulation activates a pain pathway underlying migraine headache. Pain. 2007;130:166–176. doi: 10.1016/j.pain.2007.03.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Levy D, Burstein R, Strassman AM. Mast cell involvement in the pathophysiology of migraine headache: a hypothesis. Headache. 2006;46(Suppl 1):S13–S18. doi: 10.1111/j.1526-4610.2006.00485.x. [DOI] [PubMed] [Google Scholar]
- 19.Grant AD, Cottrell GS, Amadesi S, et al. Protease-activated receptor 2 sensitizes the transient receptor potential vanilloid 4 ion channel to cause mechanical hyperalgesia in mice. J Physiol. 2007;578:715–733. doi: 10.1113/jphysiol.2006.121111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Sipe WE, Brierley SM, Martin CM, et al. Transient receptor potential vanilloid 4 mediates protease activated receptor 2-induced sensitization of colonic afferent nerves and visceral hyperalgesia. Am J Physiol Gastrointest Liver Physiol. 2008;294:G1288–G1298. doi: 10.1152/ajpgi.00002.2008. [DOI] [PubMed] [Google Scholar]
- 21.Cenac N, Altier C, Chapman K, et al. Transient receptor potential vanilloid-4 has a major role in visceral hypersensitivity symptoms. Gastroenterology. 2008;135:937–46. e1–2. doi: 10.1053/j.gastro.2008.05.024. [DOI] [PubMed] [Google Scholar]
- 22.Watanabe H, Vriens J, Prenen J, et al. Anandamide and arachidonic acid use epoxyeicosatrienoic acids to activate TRPV4 channels. Nature. 2003;424:434–438. doi: 10.1038/nature01807. [DOI] [PubMed] [Google Scholar]
- 23.Ahn AH. On the temporal relationship between throbbing migraine pain and arterial pulse. Headache. 2010;50:1507–1510. doi: 10.1111/j.1526-4610.2010.01765.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Chen X, Alessandri-Haber N, Levine JD. Marked attenuation of inflammatory mediator-induced C-fiber sensitization for mechanical and hypotonic stimuli in TRPV4−/− mice. Mol Pain. 2007;3:31. doi: 10.1186/1744-8069-3-31. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.