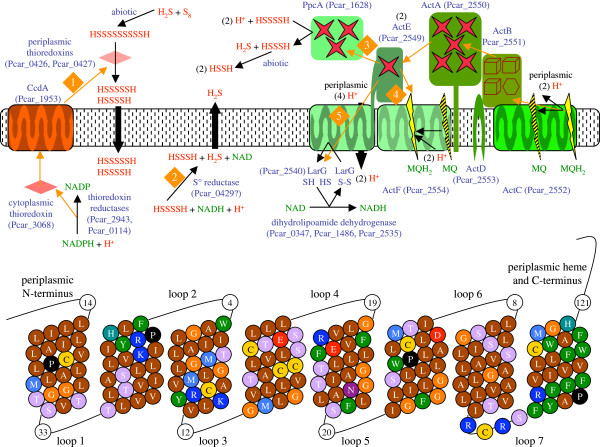
Figure 6.
Electron transfer pathways to S° and from menaquinol in P. carbinolicus. Abiotic reaction with H2S may reduce sulfur (S8) to polysulfides. Electron transfer (orange arrows) may take various pathways (numbered) to reduce polysulfides to H2S. (1) Periplasmic thioredoxins that derive electrons from NADPH may reduce polysulfides to smaller, more soluble S° species. (2) A cytoplasmic S° reductase may extract individual sulfur atoms from a chain and reduce them to H2S with NADH. (3) Alternatively, S° may be reduced by PpcA, a periplasmic triheme c7-type cytochrome that receives electrons from menaquinol (MQH2) through the Act complex. Reduction of S° by MQH2 requires reverse electron transport, but the source of energy is unknown. The ActC subunit of the Act complex oxidizes MQH2, releasing two protons to the periplasm. In a Q loop, both electrons pass to iron-sulfur cluster-binding protein ActB, pentaheme c-type cytochrome ActA, monoheme c-type cytochrome ActE, and other c-type cytochromes such as PpcA. (4) In a Q cycle, only one electron takes this route while the other passes to menaquinone (MQ) with proton uptake from the cytoplasm, perhaps involving the ActF subunit. (5) ActE could form a channel lined with eight cysteine residues for reverse electron transport to a disulfide-based cytoplasmic electron carrier such as lipoyl carrier protein LarG. Dihydrolipoamide dehydrogenase reoxidizes dihydrolipoyl-LarG and reduces NAD. The bottom part of the figure depicts in one-letter amino acid code the transmembrane segments and one cytoplasmic loop of the unique ActE of P. carbinolicus.