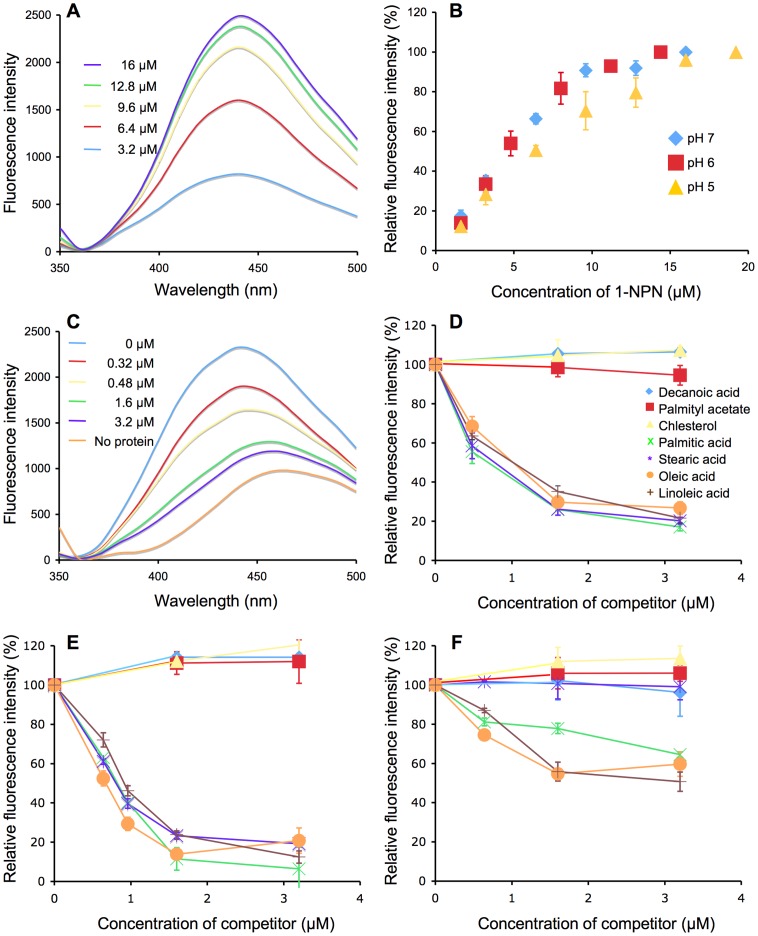
Figure 5. Binding of PregOBP56a to N-phenyl-1-naphthylamine (1-NPN) and potential ligands derived from chicken meat.
A, A typical emission spectra following the addition of 1-NPN (3.2–16 µM final concentration) to recombinant PregOBP56a (10 µg/ml of 20 mM phosphate-citrate buffer, pH 7). The emission spectra were generated following excitation at a wavelength of 337 nm. B, Binding of 1-NPN to PregOBP56a at pH 7, 6, and 5. In these experiments PregOBP56a (10 µg/ml) was incubated with various concentrations (1.6–19.2 µM) of 1-NPN and emission at 440 nm was plotted. Values are means ± standard deviation, n = 3. C, Typical emission spectra following the addition of a competitor to PregOBP56a bound to 1-NPN. PregOBP56a (10 µg/ml) in 20 mM phosphate-citrate buffer at pH 7 was pre-incubated with 16 µM of 1-NPN (final concentration). Then, palmitic acid (0–3.2 µM final concentration) was added to the reaction, and the emission spectra (excitation a wavelength of 337 nm) were recorded. D, Binding of PregOBP56a to various ligands at pH 7. For each set of data, fluorescence values at a wavelength of 440 nm were plotted as percent of that obtained in the absence of the competitor. Values are means ± standard deviation, n = 3. The competitors are shown as insets. PregOBP56a bound palmitic, stearic, oleic, and linoleic acids but not decanoic acid, palmityl acetate, or cholesterol. E, Binding of PregOBP56a to various ligands at pH 6. The results were almost identical to those in panel D. F, Binding of PregOBP56a to various ligands at pH 5. The binding affinity of stearic acid decreased to same level as decanoic acid, palmityl acetate, and cholesterol. Due to low binding to PregOBP56a, the IC50 of other test competitors was not obtained.