Figure 3. Trimerization domain without light chains.
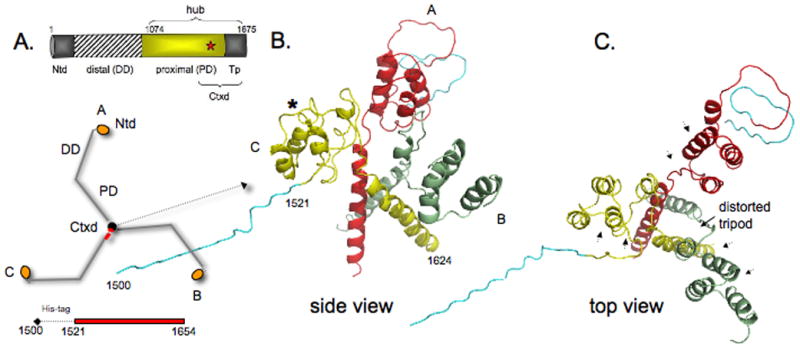
A) Domain map of clathrin heavy chain and schematic of how three identical heavy chain legs A, B, and C of clathrin form the three-legged pinwheel structure: Ntd, N-terminal domain; DD, distal domain; PD, proximal domain (light chain binding region); Tp, tripod core; Ctxd, tripod core with a segment of the proximal domain. Red star denotes cysteine-1573. Histidine-tagged Ctxd construct (1521-1654, red bar) forms a trimer. B and C) side and top views show yellow leg C (numbered 1500-1624, histidine-tag (teal) 1500-1520) is tilted down and away from the red and green legs and the 3-fold symmetry of the helix tripod is distorted. The histidine-tag and clathrin residues 1521-1527 of leg B could not be modeled because of poor density in this region. Asterisk shows Helix 7g is unfolded in the yellow leg and in the other two legs. Arrowheads in C indicate where light chain segments would be.
