Abstract
Numerous studies have shown that adiponectin confers antidiabetic effects via both insulin-like and insulin-sensitizing actions. The majority of adiponectin in circulation is derived from adipocytes; however, other tissues such as skeletal muscle can produce adiponectin. This study was designed to investigate the functional significance of adiponectin produced by skeletal muscle. We encapsulated the adiponectin gene in lipid-coated microspheres filled with octafluoropropane gas that were injected into the systemic circulation and destroyed within the microvasculature of skeletal muscle using ultrasound. We first demonstrated safe and successful targeting of luciferase and green fluorescent protein reporter genes to skeletal muscle using this approach and then confirmed efficient overexpression of adiponectin mRNA and oligomeric protein forms. Glucose tolerance test indicated that overexpression of adiponectin in skeletal muscle was able to improve glucose intolerance induced by feeding mice a high-fat diet (HFD), and this correlated with improved skeletal muscle insulin signaling. We then performed hyperinsulinemic-euglycemic clamp studies and demonstrated that adiponectin overexpression attenuated the decreases in glucose infusion rate, glucose disposal, and increase in glucose appearance induced by HFD. Ultrasound-targeted microbubble destruction (UTMD) delivery of adiponectin to skeletal muscle also enhanced serum adiponectin levels and improved hepatic insulin sensitivity. In conclusion, our data show that UTMD efficiently delivers adiponectin to skeletal muscle and that this improves insulin sensitivity and glucose homeostasis.
Keywords: adiponectin, glucose homeostasis, skeletal muscle, insulin
adiponectin exerts many beneficial physiological actions, including antidiabetic, anti-inflammatory, antiatherosclerotic, and cardioprotective effects (12, 18, 20, 42). Use of both animal models and human studies has demonstrated that adiponectin can mediate antidiabetic effects by improving glucose homeostasis via insulin-sensitizing or insulin-mimetic effects in liver and skeletal muscle (20, 42). This is significant since decreased plasma adiponectin levels, in particular the high-molecular-weight (HMW) oligomeric form, have been found in individuals with obesity and diabetes (26, 42). Indeed, mice lacking adiponectin tend to develop greater extent of glucose intolerance after high-fat diet feeding, and this can be improved by administration of adiponectin (28). Furthermore, transgenic mice overexpressing adiponectin show improved insulin sensitivity and glucose homeostasis (2, 34).
Although the majority of adiponectin in circulation is derived from adipocytes, several studies have now reported nonadipose expression of adiponectin mRNA and protein. These include various studies in skeletal muscle, such as human skeletal muscle cells grown from biopsies of vastus lateralis muscle (35), primary murine skeletal muscle (5, 14, 15, 19, 23, 25), C2C12 mouse skeletal muscle myotubes (14, 15, 19), and L6 rat skeletal muscle cells (23, 25). Furthermore, the expression of adiponectin in skeletal muscle can be influenced by factors such as hyperglycemia and hyperlipidemia (5, 15, 25), peroxisome proliferator-activator receptor-γ (PPAR-γ) activation by thiazolidinediones (1, 25), lipopolysaccharide, and proinflammatory cytokines such as interferon-γ and tumor necrosis factor-α (14, 15, 19). We have previously shown that adiponectin produced by L6 skeletal muscle cells acted in an autocrine manner to enhance both basal and insulin-stimulated glucose uptake (25). These observations are in keeping with the emerging concept that muscle itself can act as an important secretory organ and that myokines mediate important autocrine, paracrine, and endocrine effects (33, 38). However, there have been no studies to date directly examining the metabolic consequences of enhancing adiponectin production in skeletal muscle in vivo.
Ultrasound-targeted microbubble destruction (UTMD) as a means of tissue-specific gene delivery has now been established for various target tissues, such as pancreas, kidney, heart, endothelium, and skeletal muscle (4, 8–10, 22, 30, 40). The principle of this approach is to systemically infuse a chosen transgene that is precoupled to gas-filled lipid microbubbles that are burst within the microvasculature of the target tissue via application of a high-power, low-frequency ultrasound signal. This results in release of the DNA and allows for transfection of neighboring cells within the tissue. In this study we used UTMD to deliver the adiponectin gene specifically to skeletal muscle of mice with high-fat diet feeding-induced obesity, insulin resistance, and glucose intolerance. We examined the functional consequences by studying whole body glucose homeostasis with the hyperinsulinemic-euglycemic clamp technique as well as assessing insulin sensitivity in muscle and liver.
MATERIALS AND METHODS
Materials.
High-fat diet (HFD) was purchased from Research Diets. The HFD provided 20% kcal from protein, 20% kcal from carbohydrate, and 60% kcal from fat compared with the control regular chow diet (LabDiets, Brentwood, MO), which provided 20% kcal from protein, 70% kcal from carbohydrate, and 10% kcal from fat. DEFINITY perflutren lipid microsphere microbubbles were from Lantheus Medical Imaging (Saint-Laurent, QC, Canada). QIAGEN Plasmid Giga Kit was from QIAGEN (Toronto, ON, Canada). [3H]glucose was from PerkinElmer (Woodbridge, ON), and human insulin (Humulin R) was from Eli Lilly (Toronto, ON). Polyclonal antibodies to phosphospecific protein kinase B (Akt) [threonine-308 (T308) and serine-473 (S473)], Akt2, the adaptor protein containing pleckstrin homology domain, phosphotyrosine binding domain, and leucine zipper motif (APPL) 1 and GAPDH as well as horseradish peroxidase-conjugated anti-rabbit-IgG were from Cell Signaling Technology (Beverly, MA). Polyclonal antibodies to adiponectin and APPL2 were produced in-house. Antibodies to adiponectin receptors 1 and 2 (AdipoR1 and AdipoR2) were a kind gift from Dr. Tony Clementz, AstraZeneca (Molndal, Sweden). Chemiluminescence reagent plus was obtained from PerkinElmer (Boston, MA), and the Luciferase Assay System was from Promega (Madison, WI). Bradford Reagent and polyvinylidene difluoride membranes were from Bio-Rad Laboratories (Burlington, ON, Canada). All other reagents used were of the highest purity available.
Experimental animals.
C57BL/6 male mice were purchased from Charles River Laboratories (Montreal, QC, Canada), housed in a temperature-controlled environment under a 12:12-h light-dark cycle, and were fed ad libitum. Animal facilities met the guidelines of the Canadian Council on Animal Care, and all protocols used were approved by the Animal Care Committee, York University. For diet-induced obesity studies, animals received HFD commencing at 6 wk of age, with corresponding age-matched controls receiving regular chow for 6 or 12 wk.
Plasmid DNA preparation and purification.
pGL3-control luciferase vector was purchased from Promega, and myogenin promoter-driven luciferase (31) was a kind gift from Dr. John C. McDermott (York University, Toronto). Full-length adiponectin (fAd) and enhanced green fluorescent protein-tagged APPL1 (eGFP-APPL1) plasmid were produced in-house. An empty pCDNA3 vector was used as an empty vector (EV) control. All vectors were first transformed into Escherichia coli followed by overnight incubation in 37°C with agitation and antibiotics. All plasmids were then purified using the QIAGEN Plasmid Giga Kit according to the manufacturer's protocol.
Ultrasound-targeted microbubble delivery.
One milligram of plasmid DNA mixed with 100 μl DEFINITY microbubbles was infused at a constant rate of 50 μl/min via catheters inserted in the left/right jugular vein of mice. A 1-MHz, 2.0-cm2 ultrasound signal (Vevo SoniGene; Visual Sonics, Toronto, ON) at 50% duty cycle (pulsed signal) was applied to each hind leg to induce microbubble destruction and transfection. In initial experiments the right hind leg was used as the control untransfected leg, and the left leg was used for luciferase or green fluorescent protein expression. All mice were monitored after the experiment for normal behavior.
Luciferase assay.
To quantitate expression of the luciferase transgene, total protein was extracted from hind leg skeletal muscle following UTMD transfection with lysis buffer (20 mm Tris·HCl, pH 7.4, 0.1% Triton X-100). Luciferase assay was performed according to the manufacturer's protocol, and luciferase activity was quantified using a luminometer (Berthold Lumat, 9501) and standardized by total protein determined by the Bradford protein assay. Relative luciferase units (RLU) for each sample were determined from triple readings and expressed as RLU per microgram protein (fold change above readings obtained using lysates from control leg muscle).
Immunofluorescent and bright-field microscopy.
Ten-micrometer cryostat sections were obtained from mouse hind leg skeletal muscle, and green fluorescence was examined using fluorescent microscopy on the Olympus BX51 confocal microscope (Olympus, Seattle, WA) with a ×10 objective. Corresponding bright-field images were also acquired.
Hyperinsulinemic-euglycemic clamp.
Whole body insulin sensitivity was determined by hyperinsulinemic-euglycemic clamp in unrestrained, conscious mice as previously described (6) 1 wk after UTMD transfection of hind leg skeletal muscle with EV or fAd plasmids. Briefly, right jugular vein and left carotid artery catheters were embedded in animals 4 days before the hyperinsulinemic-euglycemic clamp procedure. Mice were fasted for 5–6 h before commencement of the clamp procedure, weighed, and stabilized with a constant infusion of [3H]glucose tracer for 90 min. Basal levels of blood glucose were set as the average blood glucose level + 0.5 mmol/l measured at −90, −30, −20, −10, and 0 min during the stabilization period. At 0 min, a continuous intravenous infusion of purified human insulin was started and maintained for 120 min at a constant speed of 4.0 mU·kg−1·min−1. Thirty percent dextrose solution was infused at a variable rate to maintain blood glucose at the previous set basal glucose range while carotid artery blood samples were taken at 10-min intervals to monitor plasma glucose concentrations. Erythrocytes from corresponding chow-fed or HFD blood donor animals were suspended in saline and reinfused in the animals at a continuous rate to minimize stress and maintain erythrocyte volume fraction. Blood samples collected during the clamp procedure and radioactive activities in serum samples were analyzed, and calculations were made based on the radioactivity readings to represent whole body glucose turnover rate, glucose disappearance rate (Rd), glucose appearance rate (Ra) and glycolytic rate.
Glucose tolerance test and analysis of muscle and liver insulin signaling.
To perform the glucose tolerance test animals were starved 5–6 h before receiving a bolus intraperitoneal injection of glucose (2 g/kg body wt). Tail vein blood samples were collected after 15, 30, 60, and 90 min, and the blood glucose level (mmol/l) was determined with a glucometer (Conture; Bayer). A bolus insulin dose of 4 U/kg body wt was injected via the tail vein of anesthetized mice, and skeletal muscle from both hind legs and liver tissue were excised 15 min following insulin injection and snap-frozen in liquid nitrogen for later analysis.
Tissue and serum analysis by Western blotting.
Skeletal muscle and liver homogenates were obtained from powderized tissue samples lysed with buffer containing phosphatase and protease inhibitors (30 mM HEPES, pH 7.4, 2.5 mM ethylene glycol tetraacetic acid, 3 mM ethylenediaminetetraacetic acid, 70 mM KCl, 20 mM β-glycerolphosphate, 20 mM NaF, 1 mM Na3VO4, 200 μM phenylmethylsulfonyl fluoride, 1 μM pepstatin A, 10 μM E-64, 1 μM leupeptin, and 0.1% Nonidet P-40). All samples were standardized by total protein and analyzed by SDS-PAGE. Serum and tissue adiponectin were determined by immunoblotting for multimeric and monomer total adiponectin under nonreducing/nondenaturing conditions and denaturing/reducing conditions, respectively, as described previously (25). Primary and secondary antibodies are prepared following dilutions indicated below: phospho-Akt (T308 and S473) (1:1,000); total Akt2 (1:1,000); phospho-insulin receptor substrate 1 (Y612) (1:1,000); AdipoR1 and AipoR2 (1:1,000); APPL1 and APPL2 (1:1,000), GAPDH (1:1,000); adiponectin (1:5,000); and anti-rabbit (1:10,000).
Statistical analysis.
Data were analyzed by Graphpad Prism 5, and statistical analysis was performed using the Student's t-test and one-way ANOVA followed by Tukey's Multiple-Comparison Test. Data are expressed as means ± SE where P < 0.05 was considered statistically significant.
RESULTS
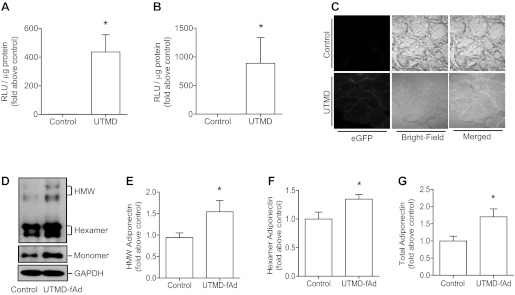
To establish UTMD as an effective method for in vivo plasmid-based gene transfer in skeletal muscle of C57BL/6 mice, we first used reporter gene constructs to evaluate the efficiency of transfection. First, a pGL3-control luciferase vector was infused, and ultrasound signal was applied to the left hind leg while the right hind leg served as a negative nontransfected control. A strong signal for luciferase activity was detected in the ultrasound-targeted leg (Fig. 1A). We next used a construct where luciferase was driven by a myogenin promoter and observed a slightly higher increase in luciferase activity in the UTMD-transfected leg (Fig. 1B). Finally, to confirm tissue-specific protein expression as a consequence of UTMD, an eGFP-APPL1 construct was used. APPL1 was not used for functional significance in this study but rather to allow for fluorescent detection of this transgene in muscle. An increase in green fluorescence was detected in UTMD-transfected skeletal muscle sections (Fig. 1C) but not in other tissues (liver, kidney, etc.) isolated from the same animal (data not shown). When these experimental conditions were used to transfect muscle with a plasmid encoding fAd, a significant increase in skeletal muscle total, HMW, and hexameric adiponectin expression was detected (Fig. 1, D–G).
Fig. 1.
Reporter gene and adiponectin gene delivery to skeletal muscle by ultrasound-targeted microbubble destruction (UTMD). We encapsulated the plasmid encoding luciferase reporter in microbubbles that were infused systemically to wild-type mice and targeted expression to skeletal muscle via applying ultrasound to one hind leg while the other hind leg served as a control. Skeletal muscle tissues were then collected, and homogenates were assayed for luciferase activity [relative luciferase units (RLU)/μg protein] (A). A similar experiment was performed using luciferase reporter gene under control of a myogenin-driven promoter (B). We then infused a plasmid containing green fluorescent protein reporter, and muscle cryostat sections were prepared to analyze green fluorescence (C). Representative collapsed “xy projections” image of skeletal muscle from enhanced green fluorescent protein (eGFP) plasmid transfection is shown. After these validation steps, we delivered the adiponectin gene to skeletal muscle using this approach, and muscle homogenates were analyzed by immunoblotting for adiponectin expression. Shown is a representative Western blot obtained from SDS-PAGE to examine adiponectin oligomers (D, top) and total adiponectin in which all samples were reduced to generate only monomer (D, bottom). fAd, full-length adiponectin. Quantitation of high molecular weight (HMW), hexameric and total adiponectin detected upon Western blotting is shown in E, F, and G, respectively. Quantitative data represent means ± SE. *P < 0.05 vs. control; n = 3–6 mice in each group.
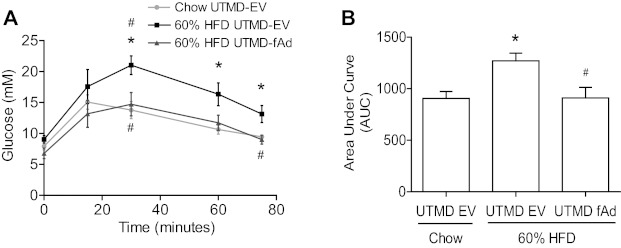
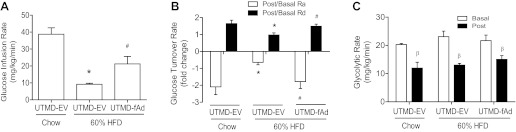
To examine if adiponectin overexpression in muscle had any functional consequences, we first conducted a glucose tolerance test in mice receiving EV or adiponectin by UTMD. We fed mice HFD, and, as expected, we found decreased glucose tolerance compared with the chow-fed age-matched control group (Fig. 2, A and B). A striking improvement in glucose tolerance was observed in HFD-fed mice with overexpression of adiponectin in skeletal muscle (Fig. 2, A and B). To study this observation in more detail, mice were subjected to a hyperinsulinemic-euglycemic clamp study that was conducted 7 days after delivery of adiponectin transgene by UTMD. As expected, a significant difference was seen in the glucose infusion rate (GIR) between chow-fed and HFD animals receiving EV UTMD transfection (Fig. 3A). Skeletal muscle overexpression of fAd significantly improved GIR, inferring improved insulin sensitivity in these mice. Whole body glucose turnover rate was calculated as the ratio of postinsulin infusion to basal levels (post:basal) for glucose Ra and glucose Rd. A significant difference in post:basal Ra (Fig. 3B) was detected between the HFD EV group compared with the chow-fed EV group, indicating decreased response to insulin-suppressed glucose production in the liver of HFD EV animals. These mice also showed reduced post:basal Rd levels compared with chow-fed EV animals (Fig. 3B) and thus a decreased response to insulin-stimulated glucose disposal in peripheral tissues. A significant correction of HFD-induced defects in Ra and Rd was achieved upon skeletal muscle fAd overexpression (Fig. 3B), demonstrating enhanced insulin responsiveness in both liver and peripheral tissues. Glycolytic rate was determined from the accumulation of 3H2O in plasma samples and is an indicator of utilization of glucose as an energy substrate. The basal and postinsulin glycolytic rates (Fig. 3C) were similar across all groups.
Fig. 2.
Glucose tolerance test (GTT). Wild-type mice were fed either commercial chow (Chow) or 60% high-fat diet (HFD) at the age of 6 wk. After 6 wk HFD, mice were subjected to either pGL3 [empty vector (EV)] or adiponectin (fAd) plasmid transfection at hind leg skeletal muscle mediated by UTMD. Seven days post-UTMD, ip glucose tolerance test (IPGTT) was performed after 5–6 h fasting on three animal groups: chow-fed UTMD-EV, 60% HF-fed UTMD-EV, and 60% HF-fed UTMD-fAd. A: IPGTT curves (mmol/l) B: area under the curve (AUC). Data represent means ± SE. *P < 0.05 vs. chow-fed UTMD-EV and #P < 0.05 vs. 60% HF-fed UTMD-EV; n = 4–5.
Fig. 3.
Hyperinsulinemic-euglycemic clamp studies following UTMD transfection in chow and diet-induced obese mice. Wild-type mice were fed either commercial chow or 60% high-fat (HF) diet at the age of 6 wk. After 12 wk HF diet, we used UTMD to deliver either pGL3 (EV) or fAd plasmid to hind leg skeletal muscles. Seven days post-UTMD, hyperinsulinemic-euglycemic clamp was performed to assess whole body glucose homeostasis on three animal groups: chow-fed UTMD-EV, 60% HFD UTMD-EV, and 60% HFD UTMD-fAd. Jugular vein and carotid artery catheters were embedded in animals 4 days before the hyperinsulinemic-euglycemic clamp procedure, and clamps were performed on animals after 5–6 h starvation. Blood samples were collected during the clamp procedure, and calculations were made based on the radioactivity readings from serum to represent whole body glucose metabolism. A: glucose infusion rate (GIR) (mg·kg−1·min−1). B: glucose turnover rate (post-to-basal ratio). Ra, glucose appearance rate; Rd, glucose disappearance rate; basal, before insulin clamp; post, after insulin clamp. C: glycolytic rate (mg·kg−1·min−1). Data represent means ± SE. *P < 0.05 vs. Chow (UTMD-EV) group and #P < 0.05 vs. 60% HF-fed UTMD-EV. βP < 0.05 vs. basal (before insulin clamp) within the same diet and treatment group; n = 4–5.
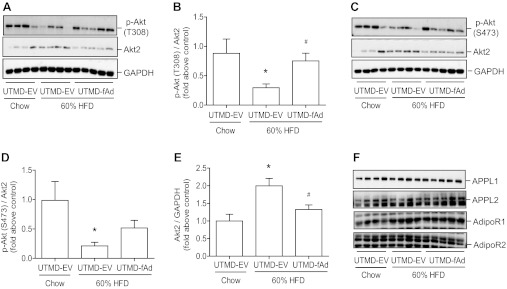
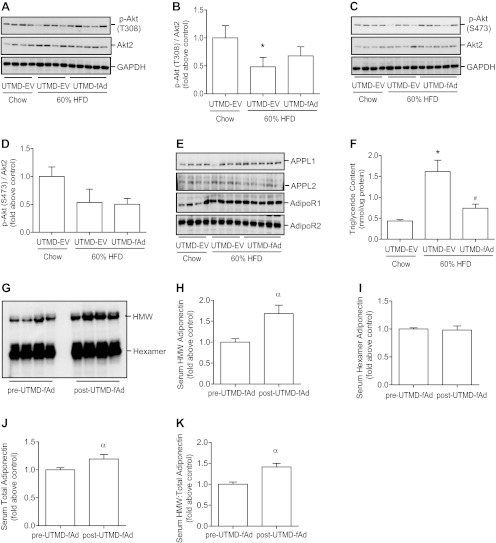
We next directly examined the influence of UTMD-mediated adiponectin overexpression on insulin sensitivity in skeletal muscle. Following a bolus intravenous injection of 4 U/kg body wt insulin, phosphorylation of Akt was examined. As expected, a decrease in insulin-stimulated Akt signaling was observed in animals fed HFD, and our data demonstrated a significant improvement in Akt signaling (T308) in skeletal muscle of HFD-fed mice after adiponectin expression (Fig. 4, A and B). An apparent improvement in insulin-stimulated phosphorylation of S473 was also observed after adiponectin was expressed in skeletal muscle of HFD-fed mice (Fig. 4, C and D). We observed that HFD induced an increase in total expression of Akt2 isoform in skeletal muscle that was reduced to similar levels to the chow-fed control group by adiponectin overexpression (Fig. 4E). Neither HFD nor adiponectin transgene expression had an effect on the expression of AdipoR1, AdipoR2, APPL1, or APPL2 (Fig. 4F).
Fig. 4.
Insulin stimulated signaling and expression of adiponectin receptor isoforms and adaptor protein containing pleckstrin homology domain, phosphotyrosine binding domain, and leucine zipper motif (APPL) isoforms in skeletal muscle. Mice were fed either commercial chow or 60% HF diet at the age of 6 wk. After 12 wk HF diet, we used UTMD to deliver either pGL3 (EV) or fAd plasmid to hind leg skeletal muscles. Seven days post-UTMD, animals were starved 5–6 h, and insulin was administered as a bolus injection (4 U/kg body wt) via tail vein. Fifteen minutes after injection skeletal muscle tissues were collected, and homogenates were prepared and then analyzed by Western blotting. Representative Western blot of phospho (p)-protein kinase B (Akt) [threonine-308 (T308, A), serine-473 (S473, C), total Akt2 (A and C)] and quantitative analysis [T308 (B), S473 (D), Akt2 (E)]. F: representative Western blot for adiponectin receptor (AdipoR) 1, AdipoR2, APPL1, and APPL2. Data represent means ± SE. *P < 0.05 vs. chow-fed UTMD-EV and #P < 0.05 vs. 60% HF-fed UTMD-EV; n = 5–6.
Data from hyperinsulinemic-euglycemic clamp studies also indicated that hepatic insulin sensitivity was improved in HFD-fed mice after adiponectin transgene expression (Fig. 3B). Therefore, we examined insulin-stimulated Akt phosphorylation in liver of the mice described above. A significant reduction in insulin-stimulated Akt phosphorylation at T308 occurred after HFD, and this was partially improved after adiponectin expression (Fig. 5, A and B). The changes observed upon study of S473 phosphorylation were less pronounced although again there was an apparent reduction in HFD-fed mice (Fig. 5, C and D). This was not altered by muscle adiponectin expression (Fig. 5, C and D). In liver we did not detect any change in Akt isoform expression among groups (Fig. 5, A and C), and neither HFD nor adiponectin transgene expression had an effect on hepatic expression of AdipoR1, AdipoR2, APPL1, or APPL2 (Fig. 5E). Because our data suggested cross talk between muscle and liver, we next examined serum adiponectin levels and found that overexpression of adiponectin transgene in skeletal muscle correlated with a small but significant increase in total serum levels of adiponectin and in particular a selective increase in the content of the oligomeric HMW form (Fig. 5, G-K). This increased adiponectin circulating level also led to a reduction of HFD-induced triglyceride accumulation in liver (Fig. 5F).
Fig. 5.
Serum adiponectin, insulin, and adiponectin signaling, lipid analysis in liver. Mice were fed either commercial chow or 60% HF diet at the age of 6 wk. After 12 wk HF diet, we used UTMD to deliver either pGL3 (EV) or fAd plasmid to hind leg skeletal muscles. Serum samples were collected pre- and post-UTMD procedure. Seven days post-UTMD, animals were starved 5–6 h, and insulin was administered as a bolus injection (4 U/kg body wt) via tail vein. Fifteen minutes after injection, liver tissue was collected, and homogenates were prepared and then analyzed by Western blotting. Representative Western blot of p-Akt [T308 (A), S473 (C), total Akt2 (A and C)] and quantitative analysis [T308 (B), S473 (D)]. E: representative Western blot for AdipoR1, AdipoR2, APPL1, and APPL2. F: liver triglyceride content (nmol/μg protein). G-K: representative Western blot and quantitative analysis of serum adiponectin level. Data represent means ± SE. *P < 0.05, chow-fed UTMD-EV vs. 60% HFD UTMD-EV, #P < 0.05, 60% HFD UTMD-EV vs. 60% HFD UTMD-fAd, and αP < 0.05 vs. pre-UTMD; n = 4–5.
DISCUSSION
This study was designed to examine the functional effects of adiponectin synthesized and secreted by skeletal muscle. The ability of adiponectin to improve insulin sensitivity and whole body glucose homeostasis in animal models of obesity and diabetes has been well documented (12, 42). Adiponectin is best known as an adipocyte-derived hormone, although there is evidence that adiponectin can be produced by a variety of tissues, including skeletal muscle (5, 14, 15, 19, 23, 25). However, the functional and physiological significance of adiponectin from nonadipose sources is not well established. We used UTMD to specifically overexpress adiponectin in skeletal muscle and examine the metabolic consequences in mice fed a HFD to induce obesity and insulin resistance.
Use of UTMD for tissue-specific gene delivery has proven effective in various tissues, including heart (3, 11, 37), pancreas (8–10), kidney (7, 17) and skeletal muscle (27, 36, 40, 43). Gene delivery via UTMD in adult mice is in fact preferable to models where the gene is eliminated or overexpressed constitutively, which are often complicated by occurrence of compensative mechanisms. Systemic delivery of plasmid DNA is possible, since its encapsulation in microbubbles serves to protect from degradation by DNases. Application of ultrasound at the tissue of choice primarily causes microbubble destruction, thus increasing local concentration of transgene yet also allowing for efficient transfection via cavitation and tissue pore formation (21, 30). In this study we first optimized our experimental protocol to establish conditions that conferred effective overexpression of luminescent and fluorescent reporter gene expression. Subsequently, this UTMD protocol was effective in enhancing muscle expression of total as well as HMW and hexameric forms of adiponectin. This clearly indicates that skeletal muscle has the molecular machinery to posttranslationally modify adiponectin and allow oligomerization to biologically active forms (24, 25, 41).
We then tested the functional metabolic significance of overexpressing adiponectin in skeletal muscle. Importantly, we first observed that overexpression of adiponectin caused a significant improvement in the plasma glucose excursion observed upon glucose challenge. To investigate this improved whole body glucose tolerance in more detail, we subjected mice to hyperinsulinemic-euglycemic clamp study (6). As expected, mice fed HFD showed a decreased GIR compared with normal chow-fed controls, indicating development of insulin resistance. In keeping with data from preliminary glucose tolerance tests, overexpressing adiponectin only in skeletal muscle of these mice caused a significant recovery of HFD-decreased GIR. Hyperinsulinemic-euglycemic clamp analysis (6) allows determination of changes in insulin-stimulated glucose uptake by peripheral tissues, including skeletal muscle (post:basal Rd) and insulin-inhibited glucose production by liver (post:basal Ra). As expected HFD induced defects in regulation of both glucose disposal and appearance compared with normal chow-fed mice. We observed a significant improvement in insulin-stimulated glucose disposal in HFD-fed mice in which UTMD was used to overexpress adiponectin in muscle. It has been estimated that 80% of serum glucose is cleared via being uptaken into skeletal muscle, indicating an important autocrine effect of adiponectin is likely in our study.
We also observed improved insulin-stimulated Akt phosphorylation in muscle of mice after adiponectin expression, which is in keeping with previous work that has shown improved basal glucose uptake and insulin-stimulated Akt phosphorylation and glucose uptake in L6 skeletal muscle cells overexpressing adiponectin (25). The improved insulin action was independent of altered expression of AdipoR1, AdipoR2, APPL1, or APPL2, four major proteins involved in adiponectin's signaling pathway in skeletal muscle, since no changes were observed between groups of animals studied here (13, 29, 39, 42). A decreased level of adiponectin has been detected in skeletal muscle of obese and diabetic animals, was enhanced when the animals were treated with rosiglitazone, and correlated with improved whole body glucose homeostasis (25). Other studies have also indicated a potentially important physiological effect of adiponectin produced by skeletal muscle. For example, elevated expression in response to proinflammatory cytokines or lipopolysaccharide has been suggested to represent a local anti-inflammatory protection mechanism (14, 19). Therefore, it is clear that skeletal muscle produces adiponectin, that this is altered in disease states, and that it may have more than one functional consequence.
Data from hyperinsulinemic-euglycemic clamp study also indicated that adiponectin overexpression in skeletal muscle improved HFD-induced defects in insulin-inhibited glucose production by liver, and analysis of Akt phosphorylation indicated improved hepatic insulin sensitivity. This suggests that cross talk between muscle and liver occurred, and, to investigate the potential role of adiponectin as the factor that mediated this cross talk, we first examined changes in serum adiponectin content. Previous estimates suggested that adiponectin derived from skeletal muscle represents only 1/174 of total circulating levels (15). Nevertheless, although a relatively small contributor to the total circulating pool, changes in muscle adiponectin levels can be of potential physiological significance. Indeed, animals with muscle-specific PPAR-γ deletion displayed reduced circulating adiponectin (16). Our analysis of serum adiponectin revealed a small but significant increase in mice in which adiponectin, as opposed to EV, was overexpressed using UTMD. Interestingly, this small change was accounted for by increased amounts of HMW adiponectin, the form that is thought to be most biologically active in liver and to mediate antidiabetic effects (32). Changes in hepatic insulin sensitivity occurred independently of changes in expression of adiponectin receptors or their adaptor protein isoforms (13, 29, 39, 42). Whether the small increase in HMW adiponectin is enough to account for the metabolic effects seen in liver is uncertain, and it is feasible that overexpression of adiponectin in skeletal muscle alters the secretome and that one or more unidentified factors mediate endocrine effects on liver.
In summary, this study is the first to use UTMD to effectively and specifically overexpress adiponectin in mouse skeletal muscle. Our data demonstrate that locally produced adiponectin can enhance muscle insulin sensitivity and glucose metabolism as well as contribute to improved whole body glucose homeostasis. This alludes to the possibility that elevating muscle adiponectin production therapeutically can be sufficient to capitalize on the antidiabetic effects of adiponectin in obese and diabetic patients.
GRANTS
Funding for this study was provided by the Canadian Institutes of Health Research via an operating grant to G. Sweeney. V. Vu was supported by a Doctoral Student Research Award from the Canadian Diabetes Association and a Graduate Scholarship from the Government of Ontario.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
AUTHOR CONTRIBUTIONS
Author contributions: V.V., Y.L., A.X., and G.S. conception and design of research; V.V., Y.L., and S.S. performed experiments; V.V., Y.L., and G.S. analyzed data; V.V. and Y.L. prepared figures; V.V. and Y.L. drafted manuscript; V.V., Y.L., A.X., and G.S. approved final version of manuscript; V.V., Y.L. and G.S. interpreted results of experiments; A.X. and G.S. edited and revised manuscript.
REFERENCES
- 1. Amin RH, Mathews ST, Camp HS, Ding L, Leff T. Selective activation of PPARgamma in skeletal muscle induces endogenous production of adiponectin and protects mice from diet-induced insulin resistance. Am J Physiol Endocrinol Metab 298: E28–E37, 2010 [DOI] [PubMed] [Google Scholar]
- 2. Asterholm IW, Scherer PE. Enhanced metabolic flexibility associated with elevated adiponectin levels. Am J Pathol 176: 1364–1376, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Bekeredjian R, Chen S, Frenkel PA, Grayburn PA, Shohet RV. Ultrasound-targeted microbubble destruction can repeatedly direct highly specific plasmid expression to the heart. Circulation 108: 1022–1026, 2003 [DOI] [PubMed] [Google Scholar]
- 4. Bekeredjian R, Grayburn PA, Shohet RV. Use of ultrasound contrast agents for gene or drug delivery in cardiovascular medicine. J Am Coll Cardiol 45: 329–335, 2005 [DOI] [PubMed] [Google Scholar]
- 5. Bonnard C, Durand A, Vidal H, Rieusset J. Changes in adiponectin, its receptors and AMPK activity in tissues of diet-induced diabetic mice. Diabetes Metab 34: 52–61, 2008 [DOI] [PubMed] [Google Scholar]
- 6. Charbonneau A, Marette A. Inducible nitric oxide synthase induction underlies lipid-induced hepatic insulin resistance in mice: potential role of tyrosine nitration of insulin signaling proteins. Diabetes 59: 861–871, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Chen HY, Huang XR, Wang W, Li JH, Heuchel RL, Chung AC, Lan HY. The protective role of Smad7 in diabetic kidney disease: mechanism and therapeutic potential. Diabetes 60: 590–601, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Chen S, Ding J, Yu C, Yang B, Wood DR, Grayburn PA. Reversal of streptozotocin-induced diabetes in rats by gene therapy with betacellulin and pancreatic duodenal homeobox-1. Gene Ther 14: 1102–1110, 2007 [DOI] [PubMed] [Google Scholar]
- 9. Chen S, Ding JH, Bekeredjian R, Yang BZ, Shohet RV, Johnston SA, Hohmeier HE, Newgard CB, Grayburn PA. Efficient gene delivery to pancreatic islets with ultrasonic microbubble destruction technology. Proc Natl Acad Sci USA 103: 8469–8474, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Chen S, Shimoda M, Wang MY, Ding J, Noguchi H, Matsumoto S, Grayburn PA. Regeneration of pancreatic islets in vivo by ultrasound-targeted gene therapy. Gene Ther 17: 1411–1420, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Chen S, Shohet RV, Bekeredjian R, Frenkel P, Grayburn PA. Optimization of ultrasound parameters for cardiac gene delivery of adenoviral or plasmid deoxyribonucleic acid by ultrasound-targeted microbubble destruction. J Am Coll Cardiol 42: 301–308, 2003 [DOI] [PubMed] [Google Scholar]
- 12. Dadson K, Liu Y, Sweeney G. Adiponectin action: a combination of endocrine and autocrine/paracrine effects. Front Cell Endocrinol 2: 1–14, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Deepa SS, Dong LQ. APPL1: role in adiponectin signaling and beyond. Am J Physiol Endocrinol Metab 296: E22–E36, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Delaigle AM, Jonas JC, Bauche IB, Cornu O, Brichard SM. Induction of adiponectin in skeletal muscle by inflammatory cytokines: in vivo and in vitro studies. Endocrinology 145: 5589–5597, 2004 [DOI] [PubMed] [Google Scholar]
- 15. Delaigle AM, Senou M, Guiot Y, Many MC, Brichard SM. Induction of adiponectin in skeletal muscle of type 2 diabetic mice: In vivo and in vitro studies. Diabetologia 49: 1311–1323, 2006 [DOI] [PubMed] [Google Scholar]
- 16. Hevener AL, He W, Barak Y, Le J, Bandyopadhyay G, Olson P, Wilkes J, Evans RM, Olefsky J. Muscle-specific Pparg deletion causes insulin resistance. Nat Med 9: 1491–1497, 2003 [DOI] [PubMed] [Google Scholar]
- 17. Hou CC, Wang W, Huang XR, Fu P, Chen TH, Sheikh-Hamad D, Lan HY. Ultrasound-microbubble-mediated gene transfer of inducible Smad7 blocks transforming growth factor-beta signaling and fibrosis in rat remnant kidney. Am J Pathol 166: 761–771, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Hui X, Lam KS, Vanhoutte PM, Xu A. Adiponectin and cardiovascular health: an update. Br J Pharmacol 165: 574–590, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Jortay J, Senou M, Delaigle A, Noel L, Funahashi T, Maeda N, Many MC, Brichard SM. Local induction of adiponectin reduces lipopolysaccharide-triggered skeletal muscle damage. Endocrinology 151: 4840–4851, 2010 [DOI] [PubMed] [Google Scholar]
- 20. Kadowaki T, Yamauchi T. Adiponectin and adiponectin receptors. Endocr Rev 26: 439–451, 2005 [DOI] [PubMed] [Google Scholar]
- 21. Klibanov AL. Microbubble contrast agents: targeted ultrasound imaging and ultrasound-assisted drug-delivery applications. Invest Radiol 41: 354–362, 2006 [DOI] [PubMed] [Google Scholar]
- 22. Korpanty G, Chen S, Shohet RV, Ding J, Yang B, Frenkel PA, Grayburn PA. Targeting of VEGF-mediated angiogenesis to rat myocardium using ultrasonic destruction of microbubbles. Gene Ther 12: 1305–1312, 2005 [DOI] [PubMed] [Google Scholar]
- 23. Krause MP, Liu Y, Vu V, Chan L, Xu A, Riddell MC, Sweeney G, Hawke TJ. Adiponectin is expressed by skeletal muscle fibers and influences muscle phenotype and function. Am J Physiol Cell Physiol 295: C203–C212, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Liu M, Zhou L, Xu A, Lam KS, Wetzel MD, Xiang R, Zhang J, Xin X, Dong LQ, Liu F. A disulfide-bond A oxidoreductase-like protein (DsbA-L) regulates adiponectin multimerization. Proc Natl Acad Sci USA 105: 18302–18307, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Liu Y, Chewchuk S, Lavigne C, Brule S, Pilon G, Houde V, Xu A, Marette A, Sweeney G. Functional significance of skeletal muscle adiponectin production, changes in animal models of obesity and diabetes, and regulation by rosiglitazone treatment. Am J Physiol Endocrinol Metab 297: E657–E664, 2009 [DOI] [PubMed] [Google Scholar]
- 26. Liu Y, Retnakaran R, Hanley A, Tungtrongchitr R, Shaw C, Sweeney G. Total and high molecular weight but not trimeric or hexameric forms of adiponectin correlate with markers of the metabolic syndrome and liver injury in Thai subjects. The J Clin Endocrinol Metab 92: 4313–4318, 2007 [DOI] [PubMed] [Google Scholar]
- 27. Lu QL, Liang HD, Partridge T, Blomley MJ. Microbubble ultrasound improves the efficiency of gene transduction in skeletal muscle in vivo with reduced tissue damage. Gene Ther 10: 396–405, 2003 [DOI] [PubMed] [Google Scholar]
- 28. Maeda N, Shimomura I, Kishida K, Nishizawa H, Matsuda M, Nagaretani H, Furuyama N, Kondo H, Takahashi M, Arita Y, Komuro R, Ouchi N, Kihara S, Tochino Y, Okutomi K, Horie M, Takeda S, Aoyama T, Funahashi T, Matsuzawa Y. Diet-induced insulin resistance in mice lacking adiponectin/ACRP30. Nat Med 8: 731–737, 2002 [DOI] [PubMed] [Google Scholar]
- 29. Mao X, Kikani CK, Riojas RA, Langlais P, Wang L, Ramos FJ, Fang Q, Christ-Roberts CY, Hong JY, Kim RY, Liu F, Dong LQ. APPL1 binds to adiponectin receptors and mediates adiponectin signalling and function. Nat Cell Biol 8: 516–523, 2006 [DOI] [PubMed] [Google Scholar]
- 30. Mayer CR, Geis NA, Katus HA, Bekeredjian R. Ultrasound targeted microbubble destruction for drug and gene delivery. Expert Opin Drug Deliv 5: 1121–1138, 2008 [DOI] [PubMed] [Google Scholar]
- 31. Miyake T, Alli NS, Aziz A, Knudson J, Fernando P, Megeney LA, McDermott JC. Cardiotrophin-1 maintains the undifferentiated state in skeletal myoblasts. J Biol Chem 284: 19679–19693, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Pajvani UB, Hawkins M, Combs TP, Rajala MW, Doebber T, Berger JP, Wagner JA, Wu M, Knopps A, Xiang AH, Utzschneider KM, Kahn SE, Olefsky JM, Buchanan TA, Scherer PE. Complex distribution, not absolute amount of adiponectin, correlates with thiazolidinedione-mediated improvement in insulin sensitivity. J Biol Chem 279: 12152–12162, 2004 [DOI] [PubMed] [Google Scholar]
- 33. Pedersen BK, Febbraio MA. Muscle as an endocrine organ: focus on muscle-derived interleukin-6. Physiol Rev 88: 1379–1406, 2008 [DOI] [PubMed] [Google Scholar]
- 34. Shetty S, Ramos-Roman MA, Cho YR, Brown J, Plutzky J, Muise ES, Horton JD, Scherer PE, Parks EJ. Enhanced fatty acid flux triggered by adiponectin overexpression. Endocrinology 153: 113–122, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Staiger H, Kausch C, Guirguis A, Weisser M, Maerker E, Stumvoll M, Lammers R, Machicao F, Haring HU. Induction of adiponectin gene expression in human myotubes by an adiponectin-containing HEK293 cell culture supernatant. Diabetologia 46: 956–960, 2003 [DOI] [PubMed] [Google Scholar]
- 36. Taniyama Y, Tachibana K, Hiraoka K, Aoki M, Yamamoto S, Matsumoto K, Nakamura T, Ogihara T, Kaneda Y, Morishita R. Development of safe and efficient novel nonviral gene transfer using ultrasound: enhancement of transfection efficiency of naked plasmid DNA in skeletal muscle. Gene Ther 9: 372–380, 2002 [DOI] [PubMed] [Google Scholar]
- 37. Taniyama Y, Tachibana K, Hiraoka K, Namba T, Yamasaki K, Hashiya N, Aoki M, Ogihara T, Yasufumi K, Morishita R. Local delivery of plasmid DNA into rat carotid artery using ultrasound. Circulation 105: 1233–1239, 2002 [DOI] [PubMed] [Google Scholar]
- 38. Trayhurn P, Drevon CA, Eckel J. Secreted proteins from adipose tissue and skeletal muscle: adipokines, myokines and adipose/muscle cross-talk. Arch Physiol Biochem 117: 47–56, 2011 [DOI] [PubMed] [Google Scholar]
- 39. Wang C, Xin X, Xiang R, Ramos FJ, Liu M, Lee HJ, Chen H, Mao X, Kikani CK, Liu F, Dong LQ. Yin-Yang regulation of adiponectin signaling by APPL isoforms in muscle cells. J Biol Chem 284: 31608–31615, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Wang X, Liang HD, Dong B, Lu QL, Blomley MJ. Gene transfer with microbubble ultrasound and plasmid DNA into skeletal muscle of mice: comparison between commercially available microbubble contrast agents. Radiology 237: 224–229, 2005 [DOI] [PubMed] [Google Scholar]
- 41. Wang Y, Lam KS, Yau MH, Xu A. Post-translational modifications of adiponectin: mechanisms and functional implications. Biochem J 409: 623–633, 2008 [DOI] [PubMed] [Google Scholar]
- 42. Yamauchi T, Kadowaki T. Physiological and pathophysiological roles of adiponectin and adiponectin receptors in the integrated regulation of metabolic and cardiovascular diseases. Int J Obesity 32, Suppl 7: S13–S18, 2008 [DOI] [PubMed] [Google Scholar]
- 43. Zhang Q, Wang Z, Ran H, Fu X, Li X, Zheng Y, Peng M, Chen M, Schutt CE. Enhanced gene delivery into skeletal muscles with ultrasound and microbubble techniques. Acad Radiol 13: 363–367, 2006 [DOI] [PubMed] [Google Scholar]