Fig. 7.
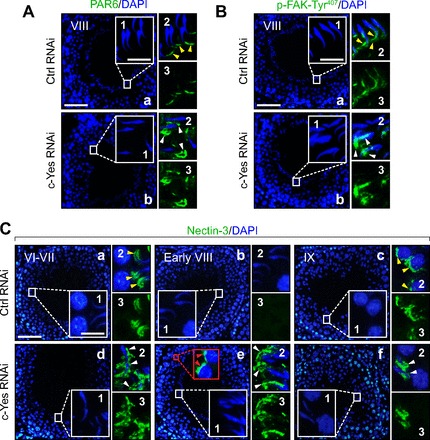
Changes in cell adhesive function at the apical ES in the seminiferous epithelium following in vivo c-Yes knockdown in testes in which defects in spermiation are mediated via an upregulation and/or mislocalization of partitioning-defective protein 6 (PAR6), p-FAK-Tyr407, and nectin-3 at the apical ES. Micrographs shown herein are rat testes from adult rats at 60–70 days of age (∼250–300 g body wt) that were transfected with either nontargeting control siRNA duplexes, as shown in A and B, image a, and C, images a–c, or c-Yes-specific siRNA duplexes for c-Yes knockdown shown in A and B, image b, and C, images d–f, and stained for PAR6 (A), p-FAK-Tyr407 (B), or nectin-3 (C). Insets 1-3 are the corresponding magnified views of the boxed areas in A and B, images a and b, and C, images a–f, and were stained with either DAPI (blue; inset 1), DAPI + either PAR6, p-FAK-Tyr407, or nectin-3 (green; inset 2), or target protein (i.e., PAR6, p-FAK-Tyr407 or nectin-3; green) alone (image 3). In control testes transfected with nontargeting control siRNA duplexes, PAR6 or p-FAK-Tyr407 was localized at the apical ES either near the tip of the head of elongated spermatids (see yellow arrowheads) or at the concave side of the elongated spermatid head (see yellow arrowheads), respectively, in stage VIII tubules, as described earlier (40, 74). However, following the knockdown of c-Yes, PAR6 and p-FAK-Tyr407 were found to be mislocalized and no longer tightly packed at the corresponding site at the apical ES as that in control testes; instead they “diffused” away from the apical ES, as annotated by white arrowheads in A and B. In rat testes that received nontargeting Ctrl RNAi duplexes in C, images a–c, the apical ES adhesion protein nectin-3 was localized at the apical ES in stage VI-VII tubules (image a), as indicated by yellow arrowheads in image a, inset 2. Nectin-3 staining was diminished considerably at the apical ES in stage VIII tubules just prior to spermiation to a level that was virtually undetectable (image b) until it reappeared again at the apical ES in stage IX tubules (image c) surrounding step 9 spermatids, as annotated by yellow arrowheads, as shown in image c, inset 2. Following c-Yes knockdown in rat testes, nectin-3 was found to be mislocalized (see white arrowheads in image d, insets 2 and 3, vs. image a, insets 2 and 3), and nectin-3 was found to be detected persistently at the apical ES even in stage VIII tubules, when its disappearance appeared to be needed to allow spermiation in a stage VIII tubule in control testes (image e vs. image b). Nectin-3 was also detected at the apical ES in step 8 spermatids; see red arrowheads in the red boxed area with enlarged view shown immediately at right. In short, nectin-3 staining was persistently found at the apical ES in c-Yes knockdown rat testes. This thus perturbed spermatid movement and adhesion, causing defects in spermiation since a transient loss of nectin-3 was necessary for spermatid movement and spermiation; see images a–c in controls. Thus, in a stage IX tubule shown in image f after spermiation had occurred in this c-Yes knockdown testis, elongated spermatids remained embedded in the seminiferous epithelium, and nectin-3 staining was also retained between Sertoli cells and the elongated spermatids, perturbing their release from the epithelium. White arrowheads in image d, inset 2, image e, inset 2, and image f, inset 2) illustrate “unwanted” and “mislocalized” nectin-3 at the apical ES. Scale bar in image a = 100 μm, which applies to image b or images b–f. Scale bar in inset 1 = 15 μm, which also applies to A–C, insets 2 and 3.
