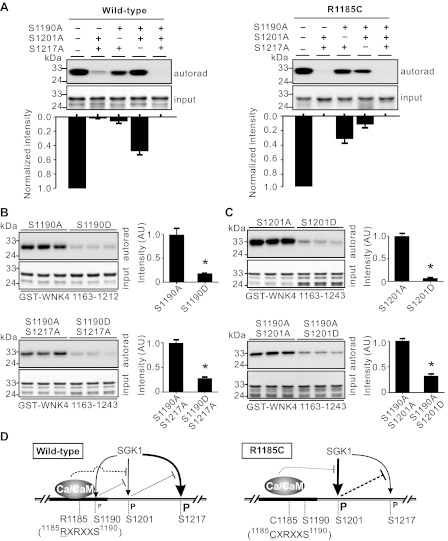
Fig. 6.
R1185C mutation affected SGK1 phosphorylation sites of WNK4 COOH terminal differently. A: SGK1 phosphorylation intensity of S1217 site was the strongest among the 3 SGK1 sites and S1190 was the weakest in WT GST-WNK4 1163–1243 fragment in the absence of Ca2+/CaM (left). In the presence of the R1185C mutation, phosphorylation of S1190 was undetectable, phosphorylation of S1201 was significantly increased, and phosphorylation of S1217 was greatly inhibited (right). The band intensities normalized to controls (without mutations in the 3 SGK1 sites) from 3 experiments are shown in the bottom panels. B: phospho-mimicking mutation S1190D suppressed S1201 phosphorylation by SGK1 in either GST-WNK4 1163–1212 or GST-WNK4 1163–1243 segment. Summaries of the band intensities from 3 experiments are shown at right. C: phospho-mimicking mutation S1201D significantly inhibited the phosphorylation of S1217 by SGK1 in GST-WNK4 1163–1243 segment. Summaries of the band intensities from 3 experiments are shown at right. *P < 0.05 between the 2 groups. D: summary of Ca2+/CaM binding and phosphorylation by SGK1 in the COOH terminal of WNK4. The degree of phosphorylation is indicated by the size of letter “P.” The thickness of lines indicates the relative strength of action.