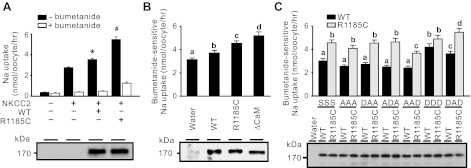
Fig. 7.
R1185C mutation in WNK4 increased Na+-K+-2Cl− cotransporter 2 (NKCC2)-mediated Na+ uptake by disrupting an inhibitory domain containing the CaM binding site. A: R1185C mutation enhanced the positive regulation of WNK4 on NKCC2-mediated Na+ uptake in X. laevis oocytes. WT WNK4 upregulated NKCC2-mediated 22Na+ uptake (top). R1185C mutation further enhanced the positive regulation of WNK4 on NKCC2 activity. Data are expressed as means ± SE from 4 independent experiments. *P < 0.05 vs. NKCC2 alone group. #P < 0.05 vs. NKCC2 + WT WNK4 group. Open bar, in the presence of 0.1 mM bumetanide; filled bar, in the absence of bumetanide. B: evaluation of the CaM binding site of WNK4 on bumetanide-sensitive 22Na+ uptake mediated by NKCC2. WT, R1185C, and WNK4 mutant with the CaM binding site (amino acids 1175–1194) deletion (ΔCaM) were used in the experiments. Data are derived from 34–49 oocytes in 3 to 4 independent experiments and are expressed as means ± SE. Unless indicated, data are not significantly different (P ≥ 0.05) and are indicated with the same letter. Equal expression of WNK4 constructs was verified by Western blot analyses with WNK4 antibody (bottom). C: evaluation of phospho-mimicking aspartate (D) mutations at S1190, S1201, and S1217 on bumetanide-sensitive 22Na+ uptake mediated by NKCC2. WT and R1185C mutant-containing mutations at the 3 phosphorylation sites are listed in single letter code in the order of their position (S, serine; A, alanine; D, aspartate). NKCC2 was expressed in all groups. Data are derived from 26–57 oocytes in 2 to 4 independent experiments and are expressed as means ± SE. The groups that are not significantly different (P ≥ 0.05) are marked with the same letter. Except for DDD group, WT is significantly different (P < 0.05) from R1185C. Bottom: representative Western blot analysis with antibody against WNK4 showing the equal expression of WNK4 WT and mutants.