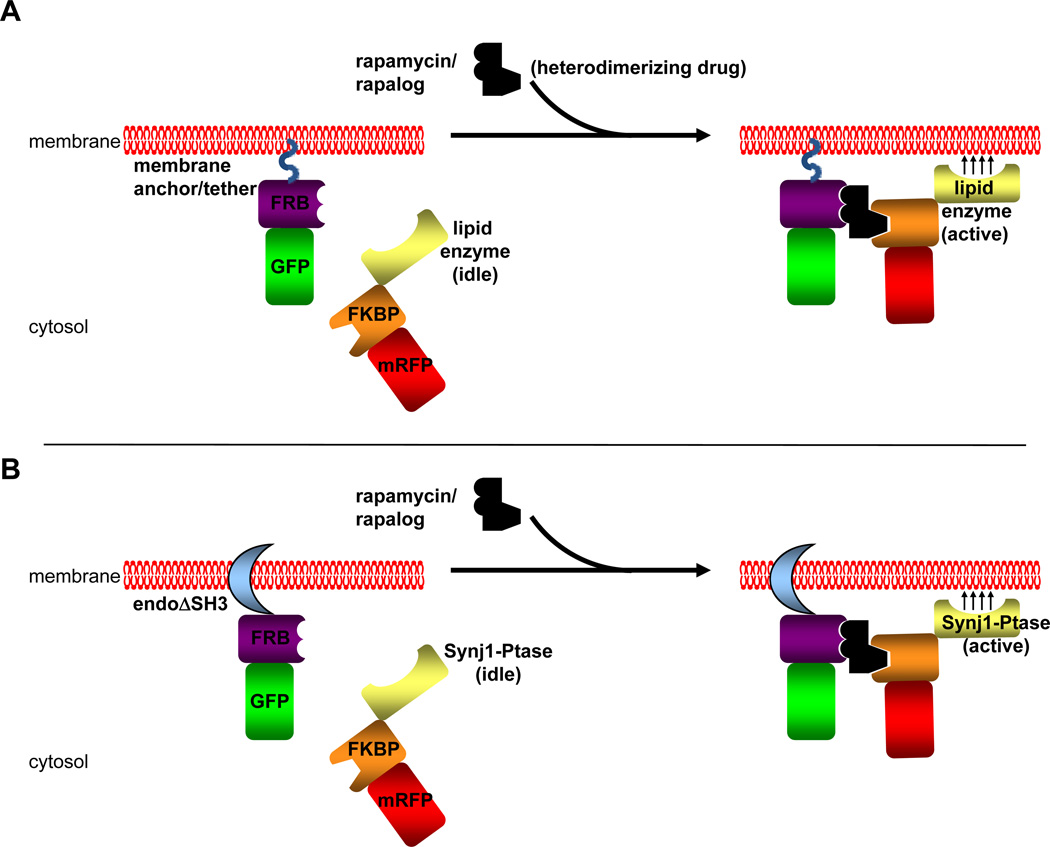
Fig. 2. Chemically Induced Dimerization Strategy.
A) A general schematic of CID using the protein modules FRB and FKBP and heterodimerizer rapamycin or one of its chemical analogs (rapalog). The FRB module is fused to a fluorescent tag (GFP in this example) and is targeted to a membrane compartment by a membrane anchor or tether. This membrane anchor/tether can be either a peptide sequence such as a myristoylation signal or a protein/protein domain that either spans or binds to a target membrane. The FKBP module is fused to another fluorescent tag (RFP in this example) and a catalytic domain of a lipid enzyme without any targeting sequences that the full length enzyme may contain. In the pre-rapamycin state (left side), the FKBP-lipid enzyme is soluble. Upon application of rapamycin or a rapalog, this heterodimerizers bring together the FRB and FKBP domains and the FKBP chimera is recruited to the membrane at which the FRB chimera is located. This brings the lipid enzyme into close proximity of the target membrane, allowing for synthesis or turnover of the desired phosphoinositide located at the target membrane. B) The CID strategy used in our study to analyze the effects of PI(4,5)P2 turnover by the endophilin-Synj1 partnership on membrane fission. In this study, the endophilin N-BAR domain was used as the membrane tether. This endophilin domain is also able to induce membrane tubulation similar to full length protein. The SH3 domain is omitted in order to avoid any recruitment of endogenous synaptojanin protein and other PRD domain-containing partners. The inositol 5-phosphatase domain from Synj1 was used to hydrolyze at PI(4,5)P2 at the endophilin-induced membrane tubules upon recruitment to these membranes by the addition of rapamycin/rapalog. The Sac1 domain of Synj1 was omitted in this study in order to study only the enzyme’s ability to dephosphorylate PI(4,5)P2 on the 5' position since the Sac1 domain has been reported to dephosphorylate several different phosphoinositide substrates in vitro (Guo et al., 1999). As with endophilin, the PRD domain of Synj1 was omitted in order to avoid any recruitment of Synj1 to membranes via interaction with SH3 domain-containing proteins.