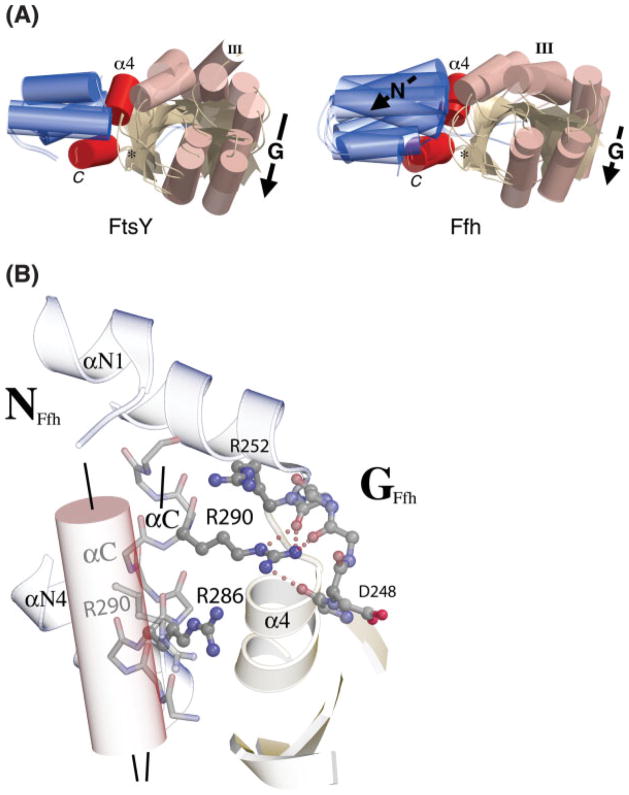
Fig. 6.
Distinct behavior of Ffh and FtsY on assembly. (A) The domain shifts of T. aquaticus Ffh and FtsY that occur during acquisition of the heterodimeric complex interface structure are different. Here, the structures of the GDP and GMPPCP (heterodimer) complexes of each protein are superimposed on a common C-terminal helix frame, which is approximately constant between the two pairs of structures. In FtsY, this frame is constant with the N domain, and the G domain shifts relative to it (Fig. 4). In Ffh, both the N- and G-domains shift relative to the helix; this is equivalent to movement of the C-terminal helix relative to the N domain on assembly into the heterodimer. In the structure of the Ffh:FtsY heterodimer the interface loops at the N/G interface (DARGG, ALLEADV) and within the G domain (I, II, III, and DAGQ) adopt symmetric configurations; their paths to acquisition of that common structure are distinct. (B) In Ffh, rearrangement of the C-terminal helix (αC) is stabilized by an interaction between Arg290 and the DARGG loop that links the position of the C-terminal helix and the sidechain of Ffh motif IV Asp248. The positions of αC in the apo (ghosted cylinder) and heterodimeric complex (backbone representation) configurations are indicated and their relative orientations highlighted by axial lines. Both Arg286 and Arg290 are conserved in the sequences of Ffh and they contribute to the formation of a basic “ladder” likely to underlay the M domain/4.5S components of the SRP in the heterodimeric state.