Table 1.
Domino Synthesis of Pentcyclic Indeno[2,1-c]quinolines 4
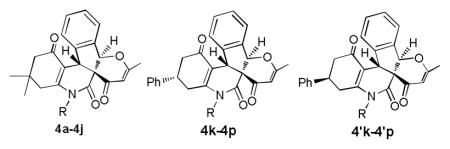
| |||||
|---|---|---|---|---|---|
| Entry | 4a | R | Timeb | Yieldc/% | 4:4′d |
| 1 | 4a | 4-Chlorophenyl (3a) | 20 | 68 | - |
| 2 | 4b | 4-Bromophenyl (3b) | 22 | 62 | - |
| 3 | 4c | 4-Iodophenyl (3c) | 25 | 58 | - |
| 4 | 4d | 3-Fluorophenyl (3d) | 25 | 44 | - |
| 5 | 4e | 3-Chlorophenyl (3e) | 26 | 52 | - |
| 6 | 4f | 3-Bromophenyl (3f) | 26 | 48 | - |
| 7 | 4g | Phenyl (3g) | 30 | 62 | - |
| 8 | 4h | 4-Methylphenyl (3h) | 32 | 68 | - |
| 9 | 4i | 3-Methylphenyl (3i) | 30 | 54 | - |
| 10 | 4j | 3-Bromo-4-methylphenyl (3j) | 28 | 50 | - |
| 11 | 4k | 4-Chlorophenyl (3k) | 28 | 58 | 75:25 |
| 12 | 4l | 4-Bromophenyl (3l) | 30 | 51 | 68:32 |
| 13 | 4m | Phenyl (3m) | 30 | 56 | 81:19 |
| 14 | 4n | 4-Methylphenyl (3n) | 36 | 60 | 89:11 |
| 15 | 4o | 3-Methylphenyl (3o) | 36 | 52 | 86:14 |
| 16 | 4p | 4-Methoxyphenyl (3p) | 30 | 64 | 64:36 |
Conditions: HOAc (1.5 mL), 80 °C, microwave heating.
Time (min).
Isolated yield.
The ratio of isomers was determined by 1H NMR.
