Abstract
Congenital pseudarthrosis of the tibia (CPT) is a rare pathology, which is usually associated with neurofibromatosis type I. The natural history of the disease is extremely unfavorable and once a fracture occurs, there is a little or no tendency for the lesion to heal spontaneously. It is challenging to treat effectively this difficult condition and its possible complications. Treatment is mainly surgical and it aims to obtain a long term bone union, to prevent limb length discrepancies, to avoid mechanical axis deviation, soft tissue lesions, nearby joint stiffness, and pathological fracture. The key to get primary union is to excise hamartomatous tissue and pathological periosteum. Age at surgery, status of fibula, associated shortening, and deformities of leg and ankle play significant role in primary union and residual challenges after primary healing. Unfortunately, none of invasive and noninvasive methods have proven their superiority. Surgical options such as intramedullary nailing, vascularized fibula graft, and external fixator, have shown equivocal success rate in achieving primary union although they are often associated with acceptable results. Amputation must be reserved for failed reconstruction, severe limb length discrepancy and gross deformities of leg and ankle. Distinct advantages, complications, and limitation of each primary treatment as well as strategies to deal with potential complications have been described. Each child with CPT must be followed up till skeletal maturity to identify and rectify residual problems after primary healing.
Keywords: Congenital pseudarthrosis of the tibia, complications, children, surgery, treatment
INTRODUCTION
The reported incidence of Congenital pseudarthrosis of the tibia (CPT) varies between 1:140,000 and 1:250,000 and bilateral forms are extremely rare.1 The tibia shows area of segmental dysplasia resulting in anterolateral bowing of the bone. The osseous dysplasia leads to a tibial nonunion and, because of tibial bowing and reduced growth in the distal tibial epiphysis, shortening of the limb usually occurs.2
The disease becomes evident within a child's first year of life. However, Andersen described a rare late onset type developing in a tibia, which is normal at birth but develops anterior bowing between the ages of 4 and 12 years.2 The natural history of the disease is extremely unfavorable and, once a fracture occurs, there is a little or no tendency for the lesion to heal spontaneously.3
Treatment of CPT is still a challenge.2,4–6 Each treatment aims to obtain a long term bony union of tibia and fibula, to prevent limb-length discrepancy, to avoid mechanical axis deviation, soft tissues lesions, nearby joint stiffness, and pathological fractures.7,8 Invasive method of treatment that includes excision of pseudarthrosis tissue including diseased periosteum with stable internal or external fixation with vascularized or nonvascularized graft is the conventional surgery for treatment of CPT. A noninvasive method, which includes electrical stimulation without surgery, is also described to enhance union. However, none of invasive or noninvasive method has yet proven its superiority. Conventional surgery is often associated with acceptable results,9,10 and amputation may be proposed in failed cases.2,11–15 Weber reported amputation of the calf in 9-14% of patients with CPT.16
Charnley (1956) first treated cases of CPT with intramedullary rod.17 Coleman added autogenous iliac bone graft to intramedullary rod for the treatment of CPT.14,18 In 1974, Ostrup et al. demonstrated the presence of active osteocytes in vascularized bone graft and their absence in nonvascularized bone graft.19,20 Taylor et al. (1975) succeeded in grafting the first vascularized fibula21 and 3 years later, the same technique was applied by Weiland et al.22,23 for treating patients with CPT. Shah et al. used cortical bone grafting with intramedullary nailing for the treatment of the CPT.24 Cortical bone grafting from contralateral tibia resist resorption better than cancellous bone hence primary union rate is higher with use of cortical bone graft.25
Since then, therapeutic progress was achieved and therapeutic protocols were implemented and improved: Bone graft associated with stable circular external fixation26–28 or intramedullary rod29,30 often results in bone union.9,10 The use of Bone Morphogenetic Protein (BMP) 7 is a therapeutic option. Despite promising preliminary results,31,32 it has nevertheless not proven its efficacy yet. Unfortunately, no treatment can guarantee good, long lasting bone union, and preserved articular function for all cases of CPT.7,26,30,33–35 Due to the lack of clarity as to the optimum treatment, the authors aimed to provide a comprehensive review on diagnosis, treatment options, and possible complications of CPT.
We aimed to describe the advantages and shortcoming of various treatment options, variables influencing the treatment of CPT, the success rate of achieving union, maintenance of union, specific complications related to pathology, and treatment. This article is prepared by a search of English literature from 1952 to 2012, using key words of congenital pseudarthrosis of the tibia, anterolateral bowing of the tibia from the PubMed database. A hand search of the references of these articles was also performed. 289 articles were retrieved in PubMed. 180 full texts and 109 abstracts were read.
RADIOGRAPHIC ASSESSMENT AND CLASSIFICATION SYSTEMS
Conventional radiographs
Various classification systems based on radiographic finding have been developed. Because of the radiographic changes during the course of the disease, standard radiographs show heterogeneous lesions. Nonunion occurs after progressive worsening of the deformity. Conventional radiographs can show thin and atrophic or wide and hypertrophic tibial bone, often with a cupped proximal and a pointed distal fragment. This false joint is often in the distal third of the shaft, but can occur at any level. The fibula is frequently affected [Figures 1 and 2].
Figure 1.
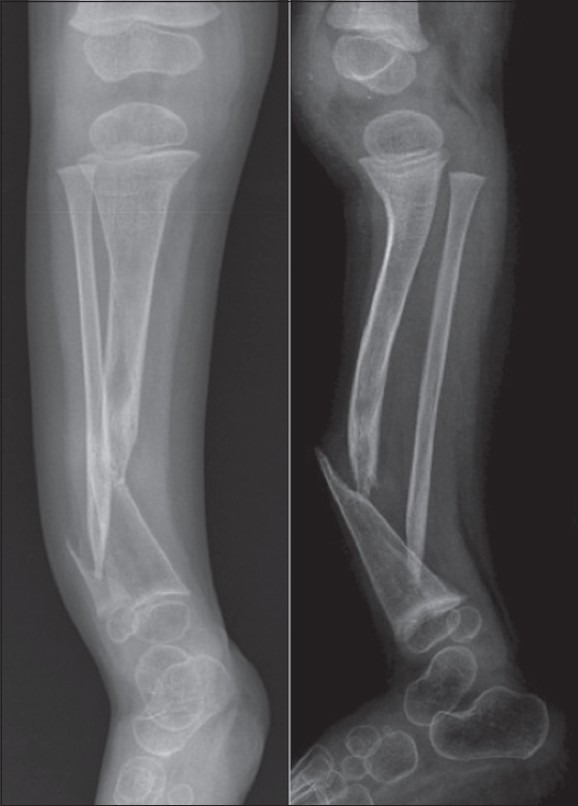
Anteroposterior and lateral radiographs of a patient with CPT. Radiographs show thin and atrophic tibial bone, with a pointed distal fragment. The false joint is in the distal third of the shaft. The fibula is affected
Figure 2.
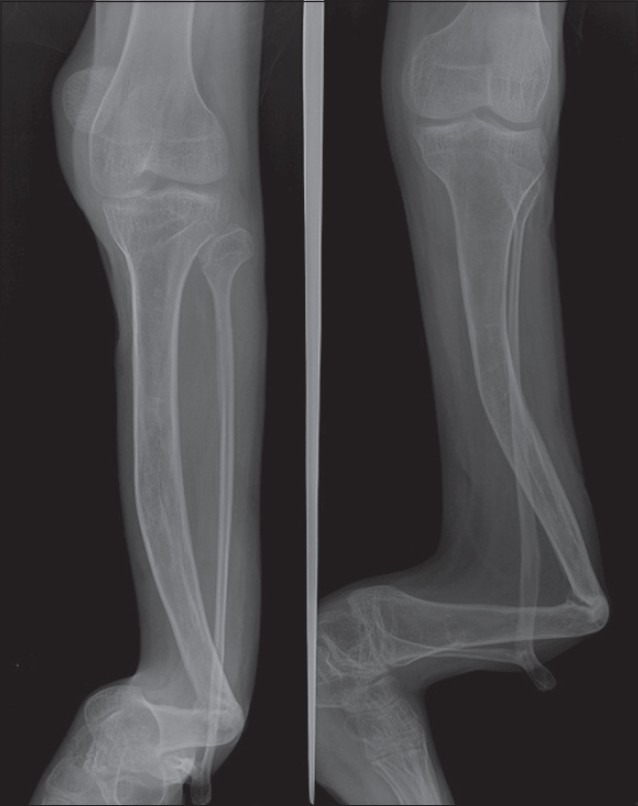
Anteroposterior radiographs of a patient with untreated CPT
Classification systems describing prognostic radiographic characteristics, natural history, therapeutic guidelines, and variables influencing the outcomes have been proposed by many different authors.
Andersen's classification
Andersen's classification of CPT (1973) differentiates the morphology of the pseudarthrosis as dysplastic, cystic, or sclerotic types, in addition to a clubfoot type that arises because of accompanying abnormalities.2 According to Andersen, neurofibromatosis is not associated with the cystic form. However, Morrissy et al. reported three cases of cystic pseudarthrosis and neurofibromatosis.36
Boyd's classification
The Boyd's classification (1982) identifies six types of CPT.6 In type I there is anterior bowing with tibial defect. Type II and III are characterized by pseudarthrosis with hour glass constrictions and bony cyst, respectively. In type IV, pseudarthrosis is present with sclerotic segments and march fracture. Type V is characterized by a complementary dysplastic fibula. In type VI, an intraosseous neurofibroma or schwannoma is evident.
Crawford's classification
Crawford's classification of CPT (1986) identifies four types of CPT all with anterolateral bowing of the tibia.5 In type I, the medullary canal is preserved and cortical thickening at the apex of the deformity might be observed; patients with this type usually have a good prognosis; some may not even have a fracture. Type II is defined by presence of thinned medullary canal, cortical thickening, and trabeculation defect. The dominant finding in type III is a cystic lesion, which may be fractured; patients with this type of pseudarthrosis tend to experience early fracture and, therefore, require early treatment. In type IV, pseudarthrosis is present with tibial and possibly fibular nonunion.
EI-Rosasy–Paley–Herzenberg's classification
El-Rosasy's et al. classification (2007) considers patient's condition at time of presentation (relation to previous treatment), radiological (atrophic or hypertrophic), and mobility of pseudarthrosis site (stiff or mobile). Three types of CPT are identified. Type I is defined by atrophic bone ends, mobile pseudarthrosis without previous surgical intervention. Type II is defined by atrophic bone ends, mobile pseudarthrosis, previous surgical intervention with or without retained hardware, while type III is defined by broad bone ends, stiff pseudarthrosis and with or without previous surgical intervention. They recommended treatment protocol according to the type of CPT.37 The drawbacks of the classification are that it does not predict prognosis neither in ascending or descending order and short-term results. The classification seems promising, but still it has to be validated in long term results and multicenter study.
Magnetic resonance imaging
Magnetic Resonance Imaging (MRI) of CPT provides valuable information on the extent of the disease and is helpful for the preoperative planning in that the borders for resection can be defined precisely. MRI findings provide more precise data on the morphology of the pseudarthrosis and the adjacent soft tissue than standard radiographs. The area of the pseudarthrosis is hyper intense on fat-suppressed and T2-weighted images and slightly hypo intense on T1-weighted images with contrast enhancement after administration of gadolinium. The periosteum in the area of the pseudarthrosis appears as a thickened soft-tissue layer. The thickened periosteum shows hyper intense signal intensity on fat-suppressed and T1-weighted contrast-enhanced images in all patients. Mahnken et al. found that MRI findings correlates well with the extent of the pseudarthrosis and of the thickened periosteum found at surgery.16 Moreover, MRI can detect deep soft-tissue neurofibromas although they are rarely found at the site of pseudarthrosis.
MRI can be recommended as an additional imaging technique that can be used with conventional radiographs in the diagnosis and followup of CPT.
Computed tomography scan
Computed Tomography scan (CT) usually confirm radiographic findings, showing osteolytic lesions containing solid tissue. The cortex is generally thinned and blown, or can no longer exist. The medullary canal is narrowed and sclerotic. The lesion can be delineated by a sclerotic rim.
Total bone scintigraphy
Bone scintigraphy shows at the level of the pseudarthrosis a slight uptake at the beginning of the dynamic venous phase and a high uptake during terminal phase.
PATHOGENESIS
In CPT, the affected tibia exhibits insufficient mechanical strength and osteogenetic capability. An abnormal highly cellular fibrovascular tissue grows at expense of the bony cortex. As it encroaches on the bony cortex it promotes incoordinate osteoclastic bone resorption that does not have a normal bone modeling purpose. Reactive changes simultaneously occur at medullary aspect leading to deposition of excess trabecular bone and hence medullary sclerosis. Excessive bone resorption accounts for intracortical cysts. Impaired vascularization can result in decreased osteogenic capabilities. The similarity of ultrastructural findings in the abnormal periosteum and in skin neurofibromas of neurofibromatosis patients may indicate a pathogenetic association of both diseases.38
HISTOPATHOLOGY
Fibrous hamartoma is the key pathology of CPT, which was shown to have low osteogenicity and high osteoclastogenicity. The soft tissue at the pseudarthrotic site is composed of variable admixture of fibrous tissue, fibrocartilage, and hyaline cartilage with evidence of enchondral ossification. Spaces and clefts are lined by a synovial-like tissue. Within the bone ends, marrow spaces are devoid of hematopoiesis. This invasive fibromatosis is located in the periosteum and between broken bone ends and surrounds the tibia causing compression, osteolysis, and persistence of pseudarthrosis.6,38,39
SURGICAL OPTIONS
Surgical treatment is not always efficient and even when bone healing seems to be obtained, new fractures can occur.8,15,26,30 The quest for durable bone healing could even deteriorate mobility and articular function.7,8,10,34 When articular function is compromised and when mechanical axis is deviated, amputation should be proposed to patients with CPT.2,1–15 Even if this solution allows fast functional recovery with the use of prosthesis, family often refuses it.14,15,23
Depending on the type of treatment, age of the patient, and severity of the CPT, the percentage of bone union varies between 31% and 100%.6,8,13–15,23,26,27,30–32,35,40–44 CPT healing rates were found to be higher when the pathological periosteum and fibromatous tissue were removed.14,15,23,45
Moreover, aesthetic and functional results are extremely variables.7,9,34 Treatment results should be evaluated once skeletal maturity is reached,6,24 growth is finished, and deformity progression has ended.7,34
Surgical options are vascularized fibular grafting alone or in association with external fixation or intramedullary nailing and amputation for failed cases.26,30 The administration of BMP is still under investigation and preliminary results are encouraging.31,32 The efficacy of these methods remains to be proven.9,26
Vascularized fibular graft
Vascularized fibular grafting, whether ipsilateral43 or preferentially contralateral,23,40,46,47 is successful as it permits initial tibia consolidation. However, many potential complications have been reported. Sometimes, secondary bone grafting is necessary to obtain bone union.23,30 Simonis et al. recommended the use of this method when limb length discrepancy is more than 5 cm and pathological tissue length is greater than 3 cm.47 New-onset fractures are not rare, and they may be observed at the graft site extremity,23,40 or in the bone graft itself.48 Other complications include malalignment (anterior bowing and valgus deformity), as no remodeling occurs in these progressive angular deformities.23,30,49,50 Valgus deformity on the donor site has also been reported.48,50,51 In order to prevent this complication, distal tibiofibular metaphyseal synostosis (Langenskiold procedure) is recommended by some authors.23,50 However, this procedure may only delay, but may not prevent, the development of ankle valgus.50
In Charnley–Williams technique the intramedullary rod in tibia was inserted along with vascularized fibular graft according to the technique described by O’Brien.52 Initial consolidation occurred in every case and no fractures were recorded.53 The goal of this original technique is to obtain bone union, by mixing propitious biological environment with the vascularized bone graft,19,20 and the intramedullary rod is responsible for stability. The intramedullary rod offers good tibial alignment and prevents refracture. Hypertrophy23,47,49 and vitality19,20 of the bone graft promote bone union.
This association seems to guarantee long term consolidation and correct tibial alignment. It may also prevent refracture in severe CPT because it mixes mechanical strength of the rod and biological quality of the graft.53 Minami et al.,48 Kanaya et al.,49 and Toh et al.28 do not share this point of view, assuming the rod could alter bone graft biology. Nevertheless, this point remains to be proven. The technique reported by Moukoko et al. is not a rescue procedure. It should be considered as a therapy of choice for initial surgical treatment of CPT, as the success of the technique needs the best biological environment.53
The high success rate of primary and secondary union are the distinct advantage of vascularised fibular graft.
The limitations of this technique are cost, technical complexity, poor protection against re-fracture, failure to correct limb length discrepancy, and deformities of leg and ankle simultaneously at time of primary surgery.
Valgus ankle deformity on the donor side, re-fracture in the graft itself, recurrent nonunion at one end of the graft site and residual limb-length discrepancy are not unusual after vascularized fibular grafting.54–69 If the procedure is combined with intramedullary nailing, it prevents re-fracture rate significantly.37,48,50,51,54,60,63,69,70
Authors preference
We reserve this procedure for failure after treatment with other modalities. Ipsilateral fibula is used for vascularized graft if the fibula is normal. If the pseudarthrosis of fibula is present, a healthy segment above pseudarthrosis can be used. We prefer distal tibia–fibula fusion, if the ipsilateral fibula is used for the surgery.
External fixation
Circular external fixation as the treatment of choice of CPT allows total resection of pathological tissue, ensures stability regardless of the amount of resected tissue, and allows extension of the member, correction of axial deformities, and full support immediately after the intervention.9,26–28,42,54,55,70
Fabry and Plawecki reported initial bone union in all patients treated with external fixation after one or more surgical procedural failure.54,70 Paley et al. (1992) reported initial union in 15 out of 16 children after one treatment, and union after one additional treatment in 1 child.42 Mean age at the time of treatment was 8 years. Boero et al. (1977) reported less enthusiastic results, as circular external fixation failed in 8 out of 21 patients with CPT in the setting of Type 1 Neurofibromatosis.27 The age at the time of surgery may have influenced bone union results. Consolidation was obtained in 12 out of 14 patients who were operated when they were 5-years old, and only in 1 out of 7 patients who were treated before 4 years of age. The European Paediatric Orthopaedic Society (EPOS) report found an overall rate of consolidation of 75.5% with mean age of 7.5 years.9 These data are close to those from Ohnishi et al., as consolidation was obtained in 86% of patients, with a mean age of 4.5 years at the time of surgery.26
Cho et al. reported re-fracture-free rate of cumulative survival of 47% at 5 years and did not change thereafter. The risk of re-fracture was significantly higher with a younger age at surgery (less than 4 years), low cross-sectional area of the healed segment, recurrence of tumor, when associated with persistent fibular pseudarthrosis, residual ankle valgus, removal of an intramedullary rod, and noncompliance with bracing.56
Fixation can correct deformities of leg and ankle and limb length discrepancy simultaneously and has a high rate of primary union in children older than age 6 years. However, the external fixator can be cumbersome for small children and it fails to prevent ankle valgus. Moreover, there is high frequency of re-fracture following external fixator and involution of the regenerate bone tissue has been observed following use of circular external fixator. Circular external fixation procedure takes a long time, is complex and associated with a high risk of infection. If the child has been protected with internal splint (intramedullary nailing) and bracing till skeletal maturity, the frequency of the re-fracture can be reduced significantly.6,10,26,36,52,55
Authors preference
We use external fixator as primary treatment in children older than 8 years of age, with significant limb length discrepancy and associated deformities of the leg. We observe that significant limb length discrepancy and deformities of the leg are more in children who present after 8 years of age. We prefer to correct limb length discrepancy, deformities of leg with achieving union of the pseudarthrosis site simultaneously. The external fixation provides better axial, angular, and rotational stability of pseudarthrosis site, which is essential for primary union. We prefer to add intramedullary nailing with external fixation primarily, as it helps to realign the tibial segments and minimize the shearing force at the pseudarthrosis site during healing. We also prefer to leave intramedullary nailing after primary healing to protect against re-fracture. We allow weight bearing early following circular external fixation application. Children can resume back to school and social activities soon after the surgery.
Intramedullary rod
The use of intramedullary rod for the treatment of CPT was suggested by Charnley in 1956;17 Coleman and Umber et al. popularized this procedure in the following years.13,18 Telescopic intramedullary rod, with or without fibular fixation, and classical rod with fibular fixation are variants of the original procedure that are associated with good results, recent studies report an union rate of 80% or more.8,24,29,30
Intramedullary rod provides stability and does not disturb distal tibial epiphysis. Growth of the tibia depends on the distal end of the rod being located proximal to the ankle joint, and ankle motion is regained in most cases.34,35,44 Intramedullary rod prevents refracture; therefore, it is inadvisable to remove the rod after union8,13,14,17,30 [Figure 3].
Figure 3.
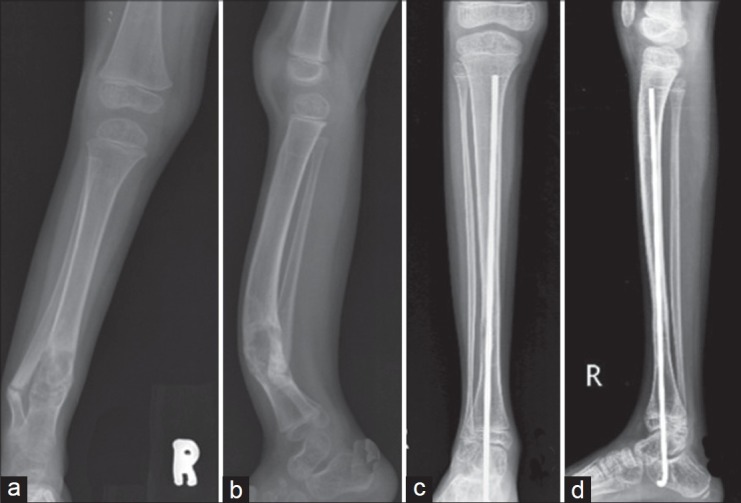
Pre (a, b) and postoperative (c, d) anteroposterior and lateral radiographs of a patient with CPT treated by intramedullary rod. Intramedullary rod provides stability and does not disturb distal tibial epiphysis
EPOS study9 reported low union rate (35%) compare to Kim et al. and Dobbs et al.,29,30 who reported union rate 80%.
Intramedullary nailing is a simple, cheap, and reproducible technique. It is effective in younger children with negligible donor site morbidity. The intramedullary nailing can also be used with vascularized fibular graft, autologous bone graft, BMPs, bisphosphonates.
Main limitations of the use of intramedullary rod include the risk for ankle stiffness secondary to long term transfixation of the tibiotalar and subtalar joints, immobilization following intramedullary rod, and re-fracture after removal of the rod. Moreover it does not always prevent ankle valgus [Figure 4]. To prevent all these, the rod must be kept till skeletal maturity. The rod must be kept out of ankle and subtalar joints once primary union is achieved.8,13,14,17,30
Figure 4.
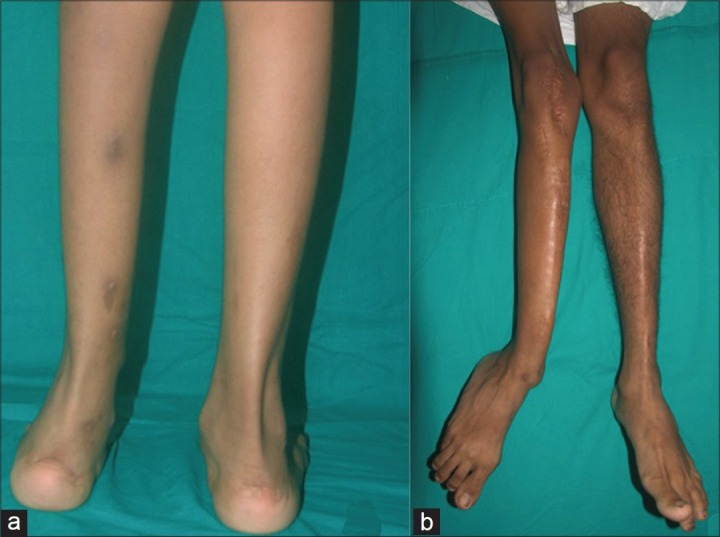
Clinical photographs showing potential complications of CPT: Ankle valgus (a) and valgus of the distal tibial shaft (b)
Authors preference
We use this method for primary treatment for younger children as it reduces the frequency of re-fracture. We prefer cortical bone graft for primary treatment as it would offer greater resistance to osteoclastic resorption. It has negligible morbidity after harvesting of the cortical graft from the contralateral tibia. Contralateral tibia must be screened to be normal clinically and radiologically before harvesting graft from opposite tibia. This technique is contraindicated in bilateral cases. Moreover, if needed, his technique can be repeated if we cannot get primary union. We keep intramedullary rod supporting full length tibia till skeletal maturity to prevent occurrence of re-fracture and deformities of leg.
Other treatment options
Dysfunction of osteoclasts and abnormal proliferation of spindle cells may be the pathogenesis of CPT with insufficient osteogenic ability.57–59 Fibrous hamartoma cells maintain some of the mesenchymal lineage cell phenotypes, but do not undergo osteoblastic differentiation in response to BMP. They are more osteoclastogenic than are tibial periosteal cells.38,39 Birke et al. recently combined the use of BMPs with bisphosphonates (pamidronate or zoledronic acid) as an adjunct to surgical intervention in eight cases. Authors hypothesized that balancing catabolic response of bisphosphonates with anabolic action of BMPs could improve healing rates. Primary healing occurred in six out of eight cases. Authors concluded key factors to achieve union in CPT include adequate resection of the dysplastic tissue, stable fixation, and the establishment of the best biologic environment for bone healing. Only a long term followup and the study of others cases can confirm or invalidate the durability of reported results.60 Periosteal graft has been used to facilitate the union. CPT is a biological as well as a mechanical problem. Many pathologic studies confirm that periosteum plays a key role in the pathogenesis of CPT combining both mechanical and biologic aspects of the disease, includes complete excision of diseased periosteum and the use of combined bone grafting and periosteal grafting.45
The efficiency of novel biological treatments (BMP, stem cells, platelet rich plasma (PRP), periosteal grafts, induced membrane technique) still has to be demonstrated, as till now all the studies were done with combination of conventional surgery and retrospective small study.61,62
Age at surgery
The results of a multicentre study conducted in 2000 by the EPOS indicate a clear correlation between age at surgery and the final outcome, with better results being achieved in the older child.7 The EPOS report recommends surgery for CPT should preferably be postponed until the age of 5 years and should not be performed on patients under the age of 3 years.
However, in a later report, Joseph et al. achieved consolidation in 92% of children under 3 years old and in 72% in children after 3 years old by intramedullary (IM) nailing with cortical bone grafting.41 More recently, Pannier et al. and Gouron et al. reported union in patients as young as 14 months with all cases stabilized by a rod crossing the ankle.63,64
Authors preference
Authors proposed that type of treatment depends on age of presentation. Treatment varies if patient is aged under 2, between 2 and 8, or over 8 years [Figure 5]. Growth abnormalities and shortening would be minimal, if we can achieve union in earlier age. We wait upto 2 years of age for surgical intervention because small size of the child may lead to difficulty in harvesting graft and also fixation of pseudarthrosis site would be poor. We consider surgical intervention of CPT after 2 years of age.
Figure 5.
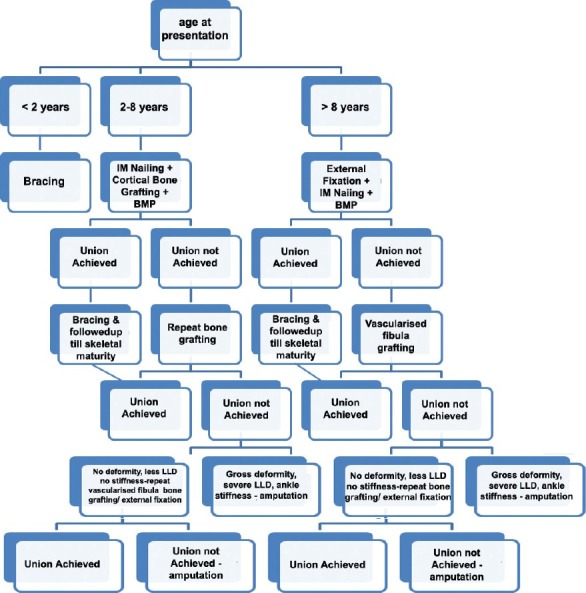
Flow chart showing treatment options according to the age at surgery: Less than 2 years, between 2 and 8 years, and over 8 years
Role of fibula
Pseudarthrosis or hypoplasia of the fibula is associated with CPT in two-thirds of the cases.65 Yet the need for fibular surgery remains controversial. Based on findings from Johnston, resection of fibular pseudarthrosis is necessary in order to achieve optimal limb alignment and union.8 If the fibula is intact, it is necessary to perform a fibular osteotomy. Dobbs et al. reported a higher rate of union in patients in whom fibular pseudarthrosis was resected as compared with those in whom fibular pseudarthrosis was not resected.30 Choi et al. classified fibula in two types: Type A (mild-A1, moderate-A2) includes a fibula of normal integrity in the presence of established atrophic-type CPT, whereas type B (mild-B1, moderate-B2, severe-B3) has atrophic-type CPT with concomitant fibular pseudarthrosis. They recommended ankle stabilization with end-to-end osteosynthesis of the fibula for mild (type B1), “4-in-1 osteosynthesis” for moderate (type B2), and distal tibiofibular fusion for severe (type B3) fibular pseudarthrosis in association with atrophic-type CPT.66
In “4-in-1 osteosynthesis” all 4 proximal and distal segments of the tibia and fibula are placed in one healing mass. It is also indicated when end-to end osteosynthesis of the fibula fails for type B1 fibular pseudarthrosis or fibular osteotomy, and shortening is necessary for a type A2 fibula. The distinct advantages of this procedure are: (a) it maximizes the cross-sectional area of healing at the pseudarthrosis level, (b) it facilitates bony healing over a wide area, (c) provides ankle stabilization and prevents proximal migration of the distal segment of the fibula, and (d) preserves ankle mobility.
Authors preference
Authors have proposed the role of fibula in management of the CPT [Figure 6].
Figure 6.
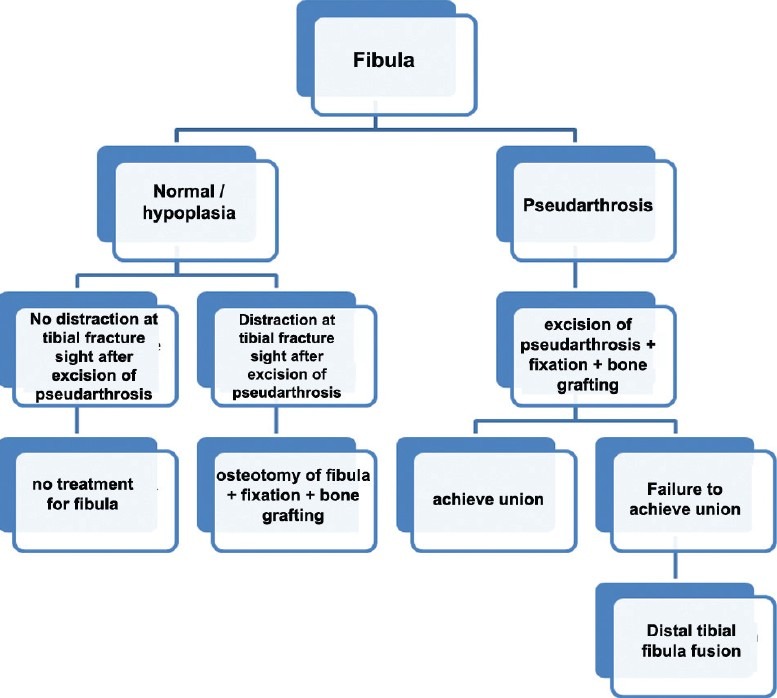
The flow chart showing the surgical options when dealing with normal and abnormal fibula (Pseudarthrosis or hypoplasia of the fibula is associated with CPT in two-thirds of the cases)
Amputation
For resistant pseudarthrosis when other extensive surgical procedures have not achieved a functional extremity, either due to persistent nonunion or due to dysfunctional angular deformity, shortening, atrophy, and stiffness, the amputation is entirely appropriate.15 While waiting for surgery, tibial alignment should be maintained with an orthosis. This is important for kids, as it allows them to walk, run, and socialize.
Authors preference
We reserve amputation after failure of two or more primary surgical procedures, gross deformities of leg and ankle, severe limb length discrepancy, recurrent re-fracture, nonfunctional limb after failed reconstruction procedures.
Ultrasound
Okada et al. reported a case of CPT of the tibia (Boyd type IV) successfully treated with low-intensity pulsed ultrasound stimulation (LIPUS) administered for 20 min/day. The treatment was continued for 1 year until solid fusion on radiographs and subsequent full-weight-bearing was achieved.67 The underlying mechanisms of action of LIPUS remain unclear. However, in experimental studies conducted in rats, LIPUS application facilitates union and increase mechanical strength of bone.68,69
Management of complications
These patients need a careful followup and residual complications need to be treated to avoid malalignment. Even patients with bone union may have compromised function secondary to residual deformities.41,71,72
Re-fracture
The frequency of re-fracture after primary union varies from 14% to 60%.10,59 Anatomic alignment of the tibia and fibula minimize the risk of re-fracture. Intramedullary rod and external bracing must be continued as effective protection against re-fractures. Despite apparently solid clinical and radiographic union, re-fracture can occur. There is a high rate of secondary nonunion following corrective osteotomy of the proximal or distal tibia.
Malalignment of the tibia
Diaphyseal malalignment of the tibia (procurvatum or valgus deformity) are progressive and do not remodel. Retention of a rod across the previous pseudarthrosis should have biomechanical benefits. The deformities of the proximal tibia can be corrected with osteotomy if the morphology of the tibia is normal with external fixator. The deformity correction with osteotomy is contraindicated through dysplastic tibia morphology, as it can lead to fresh pseudarthrosis.
Limb length discrepancy
Residual limb length discrepancy following successful union is a major problem. Growth abnormalities of the tibia, fibula, and the ipsilateral femur abnormalities are also noted with CPT, which include inclination of the proximal tibial physis, posterior bowing of the proximal third of the tibial diaphysis, proximal migration of the lateral malleolus.41 The affected tibia is slightly shorter than the normal side from beginning. Progressive shortening of the leg occurs as long as the pseudarthrosis remains ununited and also associated with repeated unsuccessful operations. Contralateral epiphyseodesis of the femur and/or tibia can be done for expected limb length discrepancy less than 5 cm at skeletal maturity. Proximal tibial lengthening can be performed in children with expected limb length discrepancy more than 5 cm at skeletal maturity. Proximal tibial lengthening by distraction osteogenesis can be performed at the metaphyseal, physeal, or subphyseal level. A good-quality regenerate without any complication can be possible with single proximal metaphyseal lengthening in children with normal morphology of proximal tibia. As, repeat lengthening and proximal dysplasic tibia were identified as significant risk factors of poor bone healing, chondrodiatasis or subphyseal lengthening of tibia or ipsilateral femoral lengthening may be better indicated when there is obvious proximal tibial dysplasia or a previous history of lengthening.73 Single proximal tibial and/or femoral lengthening and contralateral epiphyseodesis is good option for expected limb length discrepancy more than 8 cm at skeletal maturity. Femoral homolateral lengthening or contralateral distal fémoral epiphysiodesis avoid tibial complications but with the problem of knee displacement.74
Ankle valgus
Residual ankle valgus deformity compromises functional outcome.75 Progressive ankle valgus is a problematic postoperative donor-site morbidity of a vascularized fibular graft in children. To prevent this complication, tibiofibular metaphyseal synostosis (the Langenskiöld procedure) has been recommended. Correction by additional surgical procedures achieves good alignment of the lower extremity. In distal tibia corrective osteotomy, care should be taken to perform osteotomy in metaphyseal area in patient with normal morphology of distal tibia and rigidly immobilize after surgery by a cast or an Ilizarov external fixator.76 If there is enough growth remaining, the easiest and safest method to correct ankle is to perform distal tibial medial hemi-epiphysiodesis with a malleolar screw.5
Ankle stiffness
Ankle stiffness usually progressively regresses once intramedullary rod is removed from ankle. Ankle stiffness may be kept minimized with revised fixation with keeping the ankle and subtalar joint free once unequivocal, sound union of the pseudarthrosis could be achieved. Pain secondary to degenerative changes of the ankle can be treated with limitation of activity and shoe modification. Severe pain may require ankle arthrodesis.
CONCLUSION
Basic principle of excising pseudarthrosis with diseased periosteum, stable fixation, and protected weight bearing till skeletal maturity are effective method. Amputation must be reserved for failure to achieve union even after two or more good attempts, worse deformity than that produced by prosthesis. BMP holds considerable promise for the future. Healing rates in the complex forms of CPT remain uncertain and warrant larger prospective multicentre trials. Multicenter evaluation at skeletal maturity with uniform evaluation method is needed to answer the unsolved issue.
Footnotes
Source of Support: Nil
Conflict of Interest: None.
REFERENCES
- 1.Hefti F, Bollini G, Dungl P, Fixsen J, Grill F, Ippolito E, et al. Congenital pseudarthrosis of the tibia: History, etiology, classification, and epidemiologic data. J Pediatr Orthop B. 2000;9:11–5. doi: 10.1097/01202412-200001000-00003. [DOI] [PubMed] [Google Scholar]
- 2.Andersen KS. Radiological classification of congenital pseudarthrosis of the tibia. Acta Orthop Scand. 1973;44:719–27. doi: 10.3109/17453677308989112. [DOI] [PubMed] [Google Scholar]
- 3.Mahnken AH, Staatz G, Hermanns B, Gunther RW, Weber M. Congenital pseudarthrosis of the tibia in pediatric patients: MR imaging. AJR Am J Roentgenol. 2001;177:1025–9. doi: 10.2214/ajr.177.5.1771025. [DOI] [PubMed] [Google Scholar]
- 4.Gutmann DH, Aylsworth A, Carey JC, Korf B, Marks J, Pyeritz RE, et al. The diagnostic evaluation and multidisciplinary management of neurofibromatosis 1 and neurofibromatosis 2. JAMA. 1997;278:51–7. [PubMed] [Google Scholar]
- 5.Crawford AH. Neurofibromatosis in children. Acta Orthop Scand. 1986;218:1–60. [PubMed] [Google Scholar]
- 6.Boyd HB. Pathology and natural history of congenital pseudarthrosis of the tibia. Clin Orthop Relat Res. 1982;166:5–13. [PubMed] [Google Scholar]
- 7.Karol LA, Haideri NF, Halliday SE, Smitherman TB, Johnston CE., 2nd Gait analysis and muscle strength in children with congenital pseudarthrosis of the tibia: The effect of treatment. J Pediatr Orthop. 1998;18:381–6. [PubMed] [Google Scholar]
- 8.Johnston CE., 2nd Congenital pseudarthrosis of the tibia: Results of technical variations in the charnley-williams procedure. J Bone Joint Surg Am. 2002;84-A:1799–810. [PubMed] [Google Scholar]
- 9.Grill F, Bollini G, Dungl P, Fixsen J, Hefti F, Ippolito E, et al. Treatment approaches for congenital pseudarthrosis of tibia: Results of the EPOS multicenter study. J Pediatr Orthop B. 2000;9:75–89. doi: 10.1097/01202412-200004000-00002. [DOI] [PubMed] [Google Scholar]
- 10.Wientroub S, Grill F. Congenital pseudarthrosis of the tibia: Part 1. European Pediatric Orthopaedic Society multicenter study of congenital pseudoarthrosis. J Pediatr Orthop B. 2000;9:1–2. doi: 10.1097/01202412-200001000-00001. [DOI] [PubMed] [Google Scholar]
- 11.Jacobsen ST, Crawford AH, Millar EA, Steel HH. The Syme amputation in patients with congenital pseudarthrosis of the tibia. J Bone Joint Surg Am. 1983;65:533–7. [PubMed] [Google Scholar]
- 12.McCarthy RE. Amputation for congenital pseudarthrosis of the tibia. Indications and techniques. Clin Orthop Relat Res. 1982;166:58–61. [PubMed] [Google Scholar]
- 13.Umber JS, Moss SW, Coleman SS. Surgical treatment of congenital pseudarthrosis of the tibia. Clin Orthop Relat Res. 1982;166:28–33. [PubMed] [Google Scholar]
- 14.Anderson DJ, Schoenecker PL, Sheridan JJ, Rich MM. Use of an intramedullary rod for the treatment of congenital pseudarthrosis of the tibia. J Bone Joint Surg Am. 1992;74:161–8. [PubMed] [Google Scholar]
- 15.Lehman WB, Atar D, Feldman DS, Gordon JC, Grant AD. Congenital pseudoarthrosis of the tibia. J Pediatr Orthop B. 2000;9:103–7. doi: 10.1097/01202412-200004000-00005. [DOI] [PubMed] [Google Scholar]
- 16.Weber M. Neurovascular calcaneo-cutaneus pedicle graft for stump capping in congenital pseudarthrosis of the tibia: Preliminary report of a new technique. J Pediatr Orthop B. 2002;11:47–52. doi: 10.1097/00009957-200201000-00008. [DOI] [PubMed] [Google Scholar]
- 17.Charnley J. Congenital pseudarthrosis of the tibia treated by intramedullary nail. J Bone Joint Surg Am. 1956;38-A:283–90. [PubMed] [Google Scholar]
- 18.Moss UJ, Coleman S. Surgical treatment of congenital pseudarthrosis of the tibia. Clin Orthop. 1982;166:28–33. [PubMed] [Google Scholar]
- 19.Ostrup LT, Tam CS. Bone formation in a free, living bone graft transferred by microvascular anastomoses. A quantitative microscopic study using fluorochrome markers. Scand J Plast Reconstr Surg. 1975;9:101–6. doi: 10.3109/02844317509022774. [DOI] [PubMed] [Google Scholar]
- 20.Ostrup LT, Fredrickson JM. Distant transfer of a free, living bone graft by microvascular anastomoses. An experimental study. Plast Reconstr Surg. 1974;54:274–85. doi: 10.1097/00006534-197409000-00004. [DOI] [PubMed] [Google Scholar]
- 21.Taylor GI, Miller GD, Ham FJ. The free vascularized bone graft. A clinical extension of microvascular techniques. Plast Reconstr Surg. 1975;55:533–44. doi: 10.1097/00006534-197505000-00002. [DOI] [PubMed] [Google Scholar]
- 22.Weiland AJ, Daniel RK. Congenital pseudarthrosis of the tibia: Treatment with vascularized autogenous fibular grafts. A preliminary report. Johns Hopkins Med J. 1980;147:89–95. [PubMed] [Google Scholar]
- 23.Weiland AJ, Weiss AP, Moore JR, Tolo VT. Vascularized fibular grafts in the treatment of congenital pseudarthrosis of the tibia. J Bone Joint Surg Am. 1990;72:654–62. [PubMed] [Google Scholar]
- 24.Shah H, Joseph B, Siddesh ND. Congenital pseudarthrosis of the tibia treated by intramedullary rodding and cortical bone grafting-A followup study at skeletal maturity. J Pediatr Orthop. 2011;31:79–88. doi: 10.1097/BPO.0b013e318202c45d. [DOI] [PubMed] [Google Scholar]
- 25.Dodabassappa SN, Shah HH, Joseph B. Donor site morbidity following the harvesting of cortical bone graft from the tibia in children. J Child Orthop. 2010;4:417–21. doi: 10.1007/s11832-010-0277-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ohnishi I, Sato W, Matsuyama J, Yajima H, Haga N, Kamegaya M, et al. Treatment of congenital pseudarthrosis of the tibia: A multicenter study in Japan. J Pediatr Orthop. 2005;25:219–24. doi: 10.1097/01.bpo.0000151054.54732.0b. [DOI] [PubMed] [Google Scholar]
- 27.Boero S, Catagni M, Donzelli O, Facchini R, Frediani PV. Congenital pseudarthrosis of the tibia associated with neurofibromatosis-1: Treatment with Ilizarov's device. J Pediatr Orthop. 1997;17:675–84. doi: 10.1097/00004694-199709000-00019. [DOI] [PubMed] [Google Scholar]
- 28.Toh S, Harata S, Tsubo K, Inoue S, Narita S. Combining free vascularized fibula graft and the Ilizarov external fixator: Recent approaches to congenital pseudarthrosis of the tibia. J Reconstr Microsurg. 2001;17:497–509. doi: 10.1055/s-2001-17752. [DOI] [PubMed] [Google Scholar]
- 29.Kim HW, Weinstein SL. Intramedullary fixation and bone grafting for congenital pseudarthrosis of the tibia. Clin Orthop Relat Res. 2002;405:250–7. doi: 10.1097/00003086-200212000-00032. [DOI] [PubMed] [Google Scholar]
- 30.Dobbs MB, Rich MM, Gordon JE, Szymanski DA, Schoenecker PL. Use of an intramedullary rod for treatment of congenital pseudarthrosis of the tibia. A long term followup study. J Bone Joint Surg Am. 2004;86-A:1186–97. doi: 10.2106/00004623-200406000-00010. [DOI] [PubMed] [Google Scholar]
- 31.Lee FY, Sinicropi SM, Lee FS, Vitale MG, Roye DP, Jr, Choi IH. Treatment of congenital pseudarthrosis of the tibia with recombinant human bone morphogenetic protein-7 (rhBMP-7). A report of five cases. J Bone Joint Surg Am. 2006;88:627–33. doi: 10.2106/JBJS.D.02201. [DOI] [PubMed] [Google Scholar]
- 32.Fabeck L, Ghafil D, Gerroudj M, Baillon R, Delince P. Bone morphogenetic protein 7 in the treatment of congenital pseudarthrosis of the tibia. J Bone Joint Surg Br. 2006;88:116–8. doi: 10.1302/0301-620X.88B1.16619. [DOI] [PubMed] [Google Scholar]
- 33.Romanus B, Bollini G, Dungl P, Fixsen J, Grill F, Hefti F, et al. Free vascular fibular transfer in congenital pseudoarthrosis of the tibia: Results of the EPOS multicenter study. European Paediatric Orthopaedic Society (EPOS) J Pediatr Orthop B. 2000;9:90–3. doi: 10.1097/01202412-200004000-00003. [DOI] [PubMed] [Google Scholar]
- 34.Tudisco C, Bollini G, Dungl P, Fixen J, Grill F, Hefti F, et al. Functional results at the end of skeletal growth in 30 patients affected by congenital pseudoarthrosis of the tibia. J Pediatr Orthop B. 2000;9:94–102. doi: 10.1097/01202412-200004000-00004. [DOI] [PubMed] [Google Scholar]
- 35.Heikkinen ES, Poyhonen MH, Kinnunen PK, Seppanen UI. Congenital pseudarthrosis of the tibia. Treatment and outcome at skeletal maturity in 10 children. Acta Orthop Scand. 1999;70:275–82. doi: 10.3109/17453679908997807. [DOI] [PubMed] [Google Scholar]
- 36.Morrissy RT, Riseborough EJ, Hall JE. Congenital pseudarthrosis of the tibia. J Bone Joint Surg Br. 1981;63-B:367–75. doi: 10.1302/0301-620X.63B3.6790551. [DOI] [PubMed] [Google Scholar]
- 37.El-Rosasy M, Paley D, Herzenberg JE. Congenital pseudarthrosis of the tibia. In: Rozbruch SR, Ilizarov S, editors. Limb Lengthening and Reconstruction Surgery. New York: Informa Healthcare; 2007. pp. 485–93. [Google Scholar]
- 38.Lee DY, Cho TJ, Lee HR, Lee K, Moon HJ, Park MS, et al. Disturbed osteoblastic differentiation of fibrous hamartoma cell from congenital pseudarthrosis of the tibia associated with neurofibromatosis type I. Clin Orthop Surg. 2011;3:230–7. doi: 10.4055/cios.2011.3.3.230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Cho TJ, Seo JB, Lee HR, Yoo WJ, Chung CY, Choi IH. Biologic characteristics of fibrous hamartoma from congenital pseudarthrosis of the tibia associated with neurofibromatosis type 1. J Bone Joint Surg Am. 2008;90:2735–44. doi: 10.2106/JBJS.H.00014. [DOI] [PubMed] [Google Scholar]
- 40.Dormans JP, Krajbich JI, Zuker R, Demuynk M. Congenital pseudarthrosis of the tibia: Treatment with free vascularized fibular grafts. J Pediatr Orthop. 1990;10:623–8. doi: 10.1097/01241398-199009000-00010. [DOI] [PubMed] [Google Scholar]
- 41.Joseph B, Somaraju VV, Shetty SK. Management of congenital pseudarthrosis of the tibia in children under 3 years of age: Effect of early surgery on union of the pseudarthrosis and growth of the limb. J Pediatr Orthop. 2003;23:740–6. doi: 10.1097/00004694-200311000-00011. [DOI] [PubMed] [Google Scholar]
- 42.Paley D, Catagni M, Argnani F, Prevot J, Bell D, Armstrong P. Treatment of congenital pseudoarthrosis of the tibia using the Ilizarov technique. Clin Orthop Relat Res. 1992;280:81–93. [PubMed] [Google Scholar]
- 43.Coleman SS, Coleman DA. Congenital pseudarthrosis of the tibia: Treatment by transfer of the ipsilateral fibula with vascular pedicle. J Pediatr Orthop. 1994;14:156–60. doi: 10.1097/01241398-199403000-00005. [DOI] [PubMed] [Google Scholar]
- 44.Baker JK, Cain TE, Tullos HS. Intramedullary fixation for congenital pseudarthrosis of the tibia. J Bone Joint Surg Am. 1992;74:169–78. [PubMed] [Google Scholar]
- 45.Thabet AM, Paley D, Kocaoglu M, Eralp L, Herzenberg JE, Ergin ON. Periosteal grafting for congenital pseudarthrosis of the tibia: A preliminary report. Clin Orthop Relat Res. 2008;466:2981–94. doi: 10.1007/s11999-008-0556-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.de Boer HH, Verbout AJ, Nielsen HK, van der Eijken JW. Free vascularized fibular graft for tibial pseudarthrosis in neurofibromatosis. Acta Orthop Scand. 1988;59:425–9. doi: 10.3109/17453678809149396. [DOI] [PubMed] [Google Scholar]
- 47.Simonis RB, Shirali HR, Mayou B. Free vascularised fibular grafts for congenital pseudarthrosis of the tibia. J Bone Joint Surg Br. 1991;73:211–5. doi: 10.1302/0301-620X.73B2.2005141. [DOI] [PubMed] [Google Scholar]
- 48.Minami A, Ogino T, Sakuma T, Usui M. Free vascularized fibular grafts in the treatment of congenital pseudarthrosis of the tibia. Microsurgery. 1987;8:111–6. doi: 10.1002/micr.1920080302. [DOI] [PubMed] [Google Scholar]
- 49.Kanaya F, Tsai TM, Harkess J. Vascularized bone grafts for congenital pseudarthrosis of the tibia. Microsurgery. 1996;17:459–71. doi: 10.1002/(SICI)1098-2752(1996)17:8<459::AID-MICR9>3.0.CO;2-9. [DOI] [PubMed] [Google Scholar]
- 50.Kanaya K, Wada T, Kura H, Yamashita T, Usui M, Ishii S. Valgus deformity of the ankle following harvesting of a vascularized fibular graft in children. J Reconstr Microsurg. 2002;18:91–6. doi: 10.1055/s-2002-19888. [DOI] [PubMed] [Google Scholar]
- 51.Gonzalez-Herranz P, del Rio A, Burgos J, Lopez-Mondejar JA, Rapariz JM. Valgus deformity after fibular resection in children. J Pediatr Orthop. 2003;23:55–9. [PubMed] [Google Scholar]
- 52.O’Brien BM, Gumley GJ, Dooley BJ, Pribaz JJ. Folded free vascularized fibula transfer. Plast Reconstr Surg. 1988;82:311–8. doi: 10.1097/00006534-198808000-00017. [DOI] [PubMed] [Google Scholar]
- 53.Moukoko D, Chammas M, Dimeglio A. Personal Communication POSNA. 2004 [Google Scholar]
- 54.Plawecki S, Carpentier E, Lascombes P, Prevot J, Robb JE. Treatment of congenital pseudarthrosis of the tibia by the Ilizarov method. J Pediatr Orthop. 1990;10:786–90. doi: 10.1097/01241398-199011000-00015. [DOI] [PubMed] [Google Scholar]
- 55.Grill F. Treatment of congenital pseudarthrosis of tibia with the circular frame technique. J Pediatr Orthop B. 1996;5:6–16. doi: 10.1097/01202412-199605010-00002. [DOI] [PubMed] [Google Scholar]
- 56.Cho TJ, Choi IH, Lee SM, Chung CY, Yoo WJ, Lee DY, et al. Refracture after Ilizarov osteosynthesis in atrophic-type congenital pseudarthrosis of the tibia. J Bone Joint Surg Br. 2008;90:488–93. doi: 10.1302/0301-620X.90B4.20153. [DOI] [PubMed] [Google Scholar]
- 57.Ippolito E, Corsi A, Grill F, Wientroub S, Bianco P. Pathology of bone lesions associated with congenital pseudarthrosis of the leg. J Pediatr Orthop B. 2000;9:3–10. doi: 10.1097/01202412-200001000-00002. [DOI] [PubMed] [Google Scholar]
- 58.Hisaoka M, Hashimoto H, Ohguri T, Aoki T, Okamoto S, Tanaka H, et al. Congenital (infantile) pseudarthrosis of the fibula associated with osteofibrous dysplasia. Skeletal Radiol. 2004;33:545–9. doi: 10.1007/s00256-004-0759-9. [DOI] [PubMed] [Google Scholar]
- 59.Hermanns-Sachweh B, Senderek J, Alfer J, Klosterhalfen B, Buttner R, Fuzesi L, et al. Vascular changes in the periosteum of congenital pseudarthrosis of the tibia. Pathol Res Pract. 2005;201:305–12. doi: 10.1016/j.prp.2004.09.013. [DOI] [PubMed] [Google Scholar]
- 60.Birke O, Schindeler A, Ramachandran M, Cowell CT, Munns CF, Bellemore M, et al. Preliminary experience with the combined use of recombinant bone morphogenetic protein and bisphosphonates in the treatment of congenital pseudarthrosis of the tibia. J Child Orthop. 2010;4:507–17. doi: 10.1007/s11832-010-0293-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Kitoh H, Kitakoji T, Tsuchiya H, Mitsuyama H, Nakamura H, Katoh M, et al. Transplantation of marrow-derived mesenchymal stem cells and platelet-rich plasma during distraction osteogenesis - A preliminary result of three cases. Bone. 2004;35:892–8. doi: 10.1016/j.bone.2004.06.013. [DOI] [PubMed] [Google Scholar]
- 62.Granchi D, Devescovi V, Baglio SR, Leonardi E, Donzelli O, Magnani M, et al. Biological basis for the use of autologous bone marrow stromal cells in the treatment of congenital pseudarthrosis of the tibia. Bone. 2010;46:780–8. doi: 10.1016/j.bone.2009.10.044. [DOI] [PubMed] [Google Scholar]
- 63.Pannier SB, Topouchian V. Membrane induite et greffe spongieuse dans le traitement de la pseudarthrose congénitale de jambe chez l’enfant: Résultats préliminaires à propose de 3 cas. Rev Chir Orthop Reparatrice Appar Mot. 2007;93(Suppl):106. [Google Scholar]
- 64.Gouron R, Deroussen F, Juvet M, Ursu C, Plancq MC, Collet LM. Early resection of congenital pseudarthrosis of the tibia and successful reconstruction using the Masquelet technique. J Bone Joint Surg. 2011;93:552–4. doi: 10.1302/0301-620X.93B4.25826. [DOI] [PubMed] [Google Scholar]
- 65.Keret D, Bollini G, Dungl P, Fixsen J, Grill F, Hefti F, et al. The fibula in congenital pseudoarthrosis of the tibia: The EPOS multicenter study. J Pediatr Orthop B. 2000;9:69–74. doi: 10.1097/01202412-200004000-00001. [DOI] [PubMed] [Google Scholar]
- 66.Choi IH, Lee SJ, Moon HJ, Cho TJ, Yoo WJ, Chung CY, et al. “4-in-1 osteosynthesis” for atrophic-type congenital pseudarthrosis of the tibia. J Pediatr Orthop. 2011;31:697–704. doi: 10.1097/BPO.0b013e318221ebce. [DOI] [PubMed] [Google Scholar]
- 67.Okada K, Miyakoshi N, Takahashi S, Ishigaki S, Nishida J, Itoi E. Congenital pseudoarthrosis of the tibia treated with low-intensity pulsed ultrasound stimulation (LIPUS) Ultrasound Med Biol. 2003;29:1061–4. doi: 10.1016/s0301-5629(03)00906-2. [DOI] [PubMed] [Google Scholar]
- 68.Duarte LR. The stimulation of bone growth by ultrasound. Arch Orthop Trauma Surg. 1983;101:153–9. doi: 10.1007/BF00436764. [DOI] [PubMed] [Google Scholar]
- 69.Wang SJ, Lewallen DG, Bolander ME, Chao EY, Ilstrup DM, Greenleaf JF. Low intensity ultrasound treatment increases strength in a rat femoral fracture model. J Orthop Res. 1994;12:40–7. doi: 10.1002/jor.1100120106. [DOI] [PubMed] [Google Scholar]
- 70.Fabry G, Lammens J, Van Melkebeek J, Stuyck J. Treatment of congenital pseudarthrosis with the Ilizarov technique. J Pediatr Orthop. 1988;8:67–70. doi: 10.1097/01241398-198801000-00016. [DOI] [PubMed] [Google Scholar]
- 71.Inan M, El Rassi G, Riddle EC, Kumar SJ. Residual deformities following successful initial bone union in congenital pseudoarthrosis of the tibia. J Pediatr Orthop. 2006;26:393–9. doi: 10.1097/01.bpo.0000217716.64986.f0. [DOI] [PubMed] [Google Scholar]
- 72.Kristiansen LP, Steen H, Terjesen T. Residual challenges after healing of congenital pseudarthrosis in the tibia. Clin Orthop Relat Res. 2003;414:228–37. doi: 10.1097/01.blo.0000076800.53006.c9. [DOI] [PubMed] [Google Scholar]
- 73.Cho TJ, Choi IH, Lee KS, Lee SM, Chung CY, Yoo WJ, et al. Proximal tibial lengthening by distraction osteogenesis in congenital pseudarthrosis of the tibia. J Pediatr Orthop. 2007;27:915–20. doi: 10.1097/bpo.0b013e31815a6058. [DOI] [PubMed] [Google Scholar]
- 74.Bitan FR, Padovani JP, Finidori G, Touzet P. Pseudarthrose congénitale du tibia et du péroné chez l’enfant. Résultats du traitement de 18 cas par enclouage et greffe. Rev Chir Orthop. 1987;73:552–60. [PubMed] [Google Scholar]
- 75.Fragniere B, Wicart P, Mascard E, Dubousset J. Prevention of ankle valgus after vascularized fibular grafts in children. Clin Orthop Relat Res. 2003;408:245–51. doi: 10.1097/00003086-200303000-00032. [DOI] [PubMed] [Google Scholar]
- 76.Guidera KJ, Raney EM, Ganey T, Albani W, Pugh L, Ogden JA. Ilizarov treatment of congenital pseudarthroses of the tibia. J Pediatr Orthop. 1997;17:668–74. doi: 10.1097/00004694-199709000-00018. [DOI] [PubMed] [Google Scholar]


