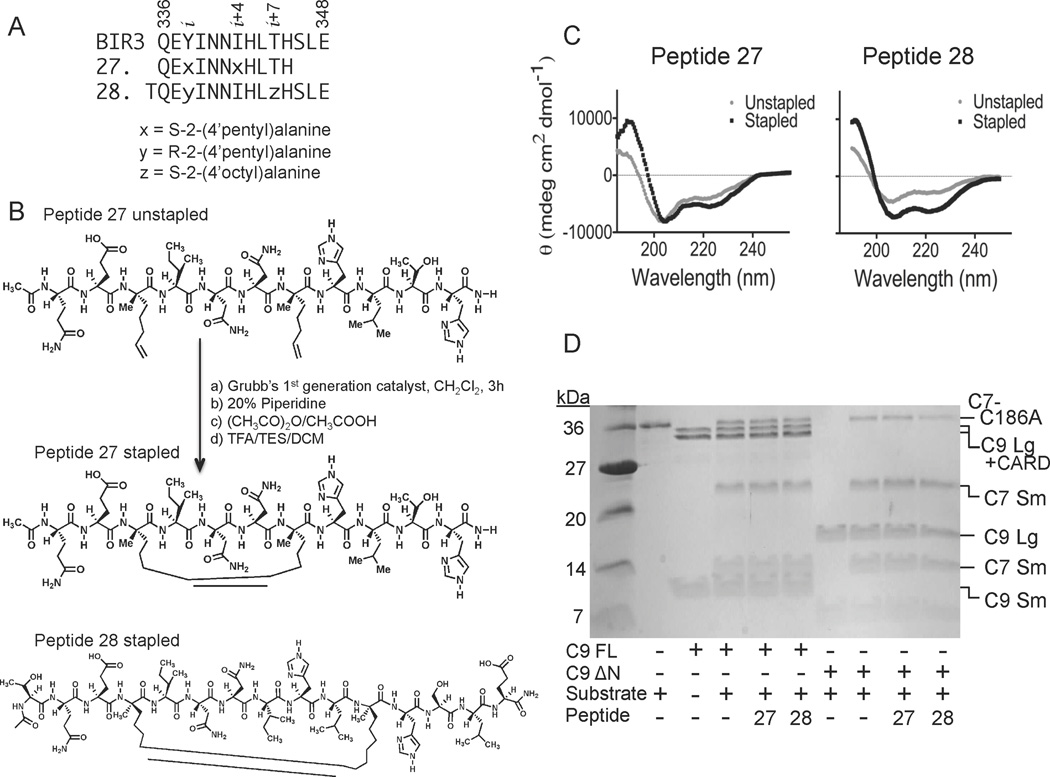
Figure 5.
Properties of aliphatically-stapled peptides. (A) Sequences of BIR3 and stapled Peptides 27 and 28. x, y and z represent the synthetic, non-native amino acids as listed. (B) Ring-closing metathesis reaction performed on the unstapled Peptide 27 to form the aliphatic stapled Peptide 27 with an 8-carbon macrocyclic linker. The structure of aliphatically stapled Peptide 28 contains an 11-carbon macrocyclic linker. (C) The CD spectra of Peptides 27 and 28 indicate a significant increase in the helicity following the formation of the aliphatic staple. (D) Stapled Peptides 27 and 28 show some inhibition of full-length caspase-9 (C9 FL) or the N-terminal CARD-domain deleted caspase-9 (C9 ΔN) to cleave a natural caspase-9 substrate, the caspase-7 zymogen (C7 C186A) to the caspase-7 large (C7 Lg) and small (C7 Sm) subunits in an in vitro cleavage assay monitored by gel mobility.