Abstract
Background
The Pediatric Heart Network trial comparing outcomes in 549 infants with single right ventricle (RV) undergoing a Norwood procedure randomized to modified Blalock-Taussig shunt (MBTS) or right ventricle-to-pulmonary artery shunt (RVPAS) found better one-year transplant-free survival in those who received RVPAS. We sought to compare the impact of shunt type on echocardiographic indices of cardiac size and function up to 14 months of age.
Methods and Results
A core laboratory measured indices of cardiac size and function from protocol exams: early after Norwood (age 22.5±13.4 days), before stage II procedure (age 4.8±1.8 months) and at 14 months (age 14.3±1.2 months). Mean RV ejection fraction was <50% at all intervals for both groups and was higher in the RVPAS group post-Norwood (49±7 vs. 44±8%, P<0.001), but was similar by 14 months. Tricuspid and neo-aortic regurgitation, diastolic function, and pulmonary artery and arch dimensions were similar in the two groups at all intervals. Neo-aortic annulus area (4.2±1.2 vs. 4.9±1.2 cm2/m2), systolic ejection times (214.0±29.4 vs. 231.3±28.6 msec), neo-aortic flow (6.2±2.4 vs. 9.4±3.4 L/min/m2), and peak arch velocity (1.9±0.7 vs. 2.2±0.7 m/sec) were lower at both interstage exams in RVPAS compared to MBTS (P<0.001 for all), but all were similar at 14 months.
Conclusion
Indices of cardiac size and function after the Norwood procedure are similar for MBTS and RVPAS by 14 months of age. Interstage differences between shunt types can likely be explained by the physiology created when the shunts are in place rather than by intrinsic differences in cardiac function.
Clinical Trial Registration #
NCT00115934, URL: http://clinicaltrials.gov/ct2/show/NCT00115934
Keywords: hypoplastic left heart syndrome, Norwood, echocardiography, single ventricle
Introduction
The Norwood procedure1 is used to palliate neonates with hypoplastic left heart syndrome (HLHS) and other single right ventricle (RV) anomalies. Two techniques are presently used to provide pulmonary blood flow in this operation. The modified Blalock-Taussig shunt (MBTS) connects the innominate or carotid artery to the pulmonary artery. The right ventricle-to-pulmonary artery shunt (RVPAS) connects the right ventricle directly to the pulmonary artery.2 Both use a valveless tube graft for these connections. There are potential advantages and disadvantages to each shunt type.3 The Pediatric Heart Network4 Single Ventricle Reconstruction (SVR) trial compared outcomes in 549 infants undergoing a Norwood procedure randomized to either MBTS or RVPAS at 15 North American centers.5 The primary result of the trial found better one-year transplant-free survival in subjects who received a RVPAS compared to those who had a MBTS.
As part of the SVR trial, 2-dimensional (2D) echocardiography was evaluated by a central core laboratory. Echocardiographic indices were measured to assess the effect of MBTS versus RVPAS at 4 stages during the trial (baseline pre-operative exam, early after the Norwood procedure, immediately prior to the stage II procedure, and at 14 months of age). The primary SVR trial result publication5 identified higher RV ejection fraction early after the Norwood procedure in the cohort who received a RVPAS compared to a MBTS using the bipyramidal method for estimation of RV volumes6 (as described in the Online Data Supplement). No differences in the percentage of subjects with moderate-severe tricuspid regurgitation were identified between shunt types at any stage. We sought to analyze this database to more fully characterize the SVR cohort using all collected echocardiographic indices, and specifically to 1) describe 2D baseline anatomic features and RV functional status of the cohort prior to the Norwood procedure, and 2) compare the effect of shunt type on 2D and Doppler indices of RV function, cardiac and vascular dimensions, valve annulus dimensions and function, and neo-aortic flow patterns early after the Norwood procedure, prior to the stage II procedure, and at 14 months of age.
METHODS
Study Design
The SVR study design7, primary outcome (incidence of death or cardiac transplantation at 12 months after randomization) and secondary outcomes (morbidity during hospitalizations for the Norwood and stage II procedures, unintended cardiovascular interventions and rate of serious adverse events through 12 months, and angiographically-derived pulmonary artery size prior to the stage II procedure) have been previously published.5 Secondary markers of outcome using echocardiography were also part of the study design and are summarized in the Online Data Supplement; with regard to echocardiographic measures, only RV ejection fraction and the percentage of subjects with moderate or severe tricuspid regurgitation have been previously published for the cohort.5
Echocardiographic Analysis
An echocardiography core laboratory at the Medical College of Wisconsin reviewed 2D/Doppler echocardiograms performed at each clinical center to compare indices between shunt groups at 4 pre-designated time intervals during the study: 1) baseline (prior to the Norwood procedure), 2) post-Norwood (either at time of discharge or at approximately 30 days of age if still hospitalized), 3) pre-stage II (during the pre-operative evaluation for the stage II procedure), and 4) 14 months of age (end of study visit).
Prior to launching the trial, all centers underwent training in the established protocol for performing the 2-D and Doppler measures from standard imaging planes in order to ensure collection of complete information. Studies were preferentially captured and stored digitally using DICOM format, transferred to the core lab on CD/DVD, and analyzed off-line using a digital review system (TomTec Imaging Systems GmbH, Unterschleissheim, Germany) custom programmed for electronic caliper overlay of captured images and database storage of study-specific measures. Each study was initially analyzed by a pediatric echocardiography technician specifically trained in the protocol, and all indices were reviewed and confirmed by the director of the core laboratory, an experienced pediatric cardiologist with specialization in echocardiography (PCF). The dataset of measures obtained on each subject was electronically transferred to the Data Coordinating Center (New England Research Institutes, Watertown, MA) for statistical analyses. The echocardiographic measures and derived parameters obtained at each study are described in the Online Data Supplement.
Statistical Analysis
Summary descriptive statistics of echocardiographic indices are presented by shunt type in place at the end of the Norwood operation at each interval. Distributions of echocardiographic indices were examined with normal probability plots and histograms. Continuous measures were compared between assigned shunt types with either Student’s or Welch’s t-test or a Wilcoxon rank sum test as appropriate. Categorical measures were compared between shunt types with a Fisher exact test; ordinal measures were compared with a Fisher exact test and the Mantel-Haenszel test for trend. Given the number of echocardiographic indices that were examined, the Bonferroni multiple comparisons correction was used such that two-sided P values <0.001 (<0.05/38 indices) were considered to be statistically significant. Analyses were performed in R version 2.12.0.
In order to adjust for the effect of somatic growth on the linear, area and volumetric dimensions of cardiac structures, z-scores were used when normative data were available.8 Since the neo-aortic valve is not a native structure, the dimensions of this valve annulus were expressed as z-scores using normal values for the dimensions of the native aortic valve in a normal population. These z-scores are calculated with respect to body surface area (BSA), with the power of BSA determined by prior work examining linearity between echo measures and various functions of BSA.9
RESULTS
Of the 555 patients who were randomized in the SVR trial, 6 were excluded from this analysis (5 who did not undergo Norwood surgery and 1 who withdrew one week after the Norwood procedure) resulting in 549 patients who were available for evaluation. A total of 268 infants undergoing a Norwood procedure received the MBTS and 281 infants received the RVPAS. Table 1 summarizes the expected number of patients available for echocardiography, the number of protocol echocardiograms obtained (the primary reasons for failure to obtain an echocardiogram being death or transplant, with a loss of about 10% of the expected cohort at each stage), the number of acceptable protocol echocardiograms for analysis, and average age at the time of the protocol echocardiogram at each stage throughout the trial. Of submitted echocardiographic studies, 99% were deemed acceptable by the core lab for analysis.
Table 1.
Number of echocardiograms at each interval by shunt type.
| Baseline | Post-Norwood | Pre-stage II | 14 months | |
|---|---|---|---|---|
| Expected number of echo studies | ||||
| Total | 549 | 544 | 452 | 373 |
| MBTS | 268 | 265 | 214 | 165 |
| RVPAS | 281 | 279 | 238 | 208 |
| Number of echo studies obtained* | ||||
| Total | 549 (100%) | 485 (89%) | 399 (88%) | 337 (90%) |
| MBTS | 268 (100%) | 238 (90%) | 175 (82%) | 148 (90%) |
| RVPAS | 281 (100%) | 247 (89%) | 224 (94%) | 189 (91%) |
| Number of echo studies deemed acceptable† | ||||
| Total | 542 (99%) | 481 (99%) | 397 (99%) | 334 (99%) |
| Age at echo study | ||||
| Total | 2.1±3.3 days | 22.5±13.4 days | 4.8±1.8 months | 14.3±1.2 months |
| MBTS | 2.1±3.4 days | 22.6±13.9 days | 4.9±2.1 months | 14.3±1.1 months |
| RVPAS | 2.0±3.2 days | 22.4±12.9 days | 4.7±1.5 months | 14.3±1.3 months |
Data shown as n (%) or mean±SD. MBTS=modified Blalock-Taussig shunt; RVPAS=right ventricular-to-pulmonary artery shunt
Percentage is number of echocardiograms obtained divided by the number of expected echocardiograms.
Percentage is the number of acceptable echocardiograms divided by the number of echocardiograms obtained.
Baseline Patient Characteristics
Baseline clinical characteristics, including anatomic diagnosis, number of patients with aortic atresia, number of patients with obstructed pulmonary venous return, and size of the ascending aorta measured from surgical inspection, have been previously published.5 No baseline anatomic features or RV functional indices were different between shunt types prior to surgery (Table 2). The median native ascending aorta diameter was 0.30 cm (interquartile range (IQR), 0.21–0.50 cm) with a median z-score of −3.8 (IQR, −4.5 – −2.4). A total of 375 (69%) subjects had an identifiable left ventricular cavity with 226 (42%) having a patent aortic valve and 262 (49%) having a patent mitral valve. Overall, RV ejection fraction (46±9%) and area fraction change (35±9%) were calculated to be low. Moderate or severe tricuspid regurgitation was found in only 12% of the cohort pre-operatively. Several of the measures, although not different between groups, could only be obtained in a small subset of the cohort because of inadequate/incomplete imaging (RV dP/dt, isovolumic acceleration) or because of fusion of the diastolic filling waves that commonly occurs in infants with sinus tachycardia (with resultant loss of an identifiable tricuspid inflow A and tricuspid Doppler tissue imaging A′ wave as well as E/A ratio and E′/A′ ratio calculations).
Table 2.
Baseline anatomic features and RV functional status by shunt type prior to Norwood procedure
| Pre-Norwood Variable | N | MBTS | N | RVPAS | p |
|---|---|---|---|---|---|
| Presence of left ventricle | 264 | 190 (72%) | 278 | 185 (67%) | 0.19 |
| Native ascending aortic size, cm | 255 | 0.30 (0.21,0.51) | 272 | 0.31 (0.21,0.49) | 0.96 |
| Native ascending aortic size, z-score | 255 | −3.80 (−4.54,−2.30) | 272 | −3.73 (−4.43,−2.39) | 0.70 |
| Aortic valve patency | 261 | 113 (43%) | 276 | 113 (41%) | 0.60 |
| Mitral valve patency | 263 | 131 (50%) | 277 | 131 (47%) | 0.61 |
| Mean atrial septal defect pressure gradient, mmHg* | 163 | 4.87 (2.41,7.50) | 168 | 4.55 (1.97,6.98) | 0.47 |
| RV systolic function | |||||
| RV ejection fraction % (bi-plane pyramidal) | 168 | 46±9 | 189 | 46±8 | 0.98 |
| RV % area change | 245 | 35±9 | 258 | 35±8 | 0.92 |
| RV dP/dt, mmHg/sec | 14 | 1062±430 | 10 | 1038±249 | 0.88 |
| Tissue Doppler tricuspid peak annular systolic velocity, cm/sec | 122 | 7.82±2.04 | 115 | 7.51±1.93 | 0.23 |
| RV diastolic function | |||||
| Peak tricuspid inflow E velocity, m/sec | 189 | 0.91±0.28 | 197 | 0.87±0.29 | 0.16 |
| Peak tricuspid inflow A velocity, m/sec | 64 | 0.89±0.26 | 65 | 0.80±0.19 | 0.03 |
| Tricuspid inflow E/A ratio | 64 | 0.75±0.19 | 65 | 0.77±0.26 | 0.74 |
| Tricuspid annular tissue Doppler E′ velocity, cm/sec | 124 | 12.8±4.8 | 116 | 12.4±4.5 | 0.53 |
| Tricuspid annular tissue Doppler A′ velocity, cm/sec | 32 | 10.2±1.9 | 33 | 10.1±3.0 | 0.96 |
| E/E′ ratio | 120 | 8.12±3.42 | 110 | 7.82±3.17 | 0.50 |
| Presence of pulmonary vein flow reversal | 149 | 13 (9%) | 158 | 23 (15%) | 0.15 |
| RV size and shape | |||||
| End-diastolic volume (mL)/BSA1.3 | 168 | 81.9 (68.5,99.3) | 190 | 84.5 (70.4,100.1) | 0.79 |
| End-systolic volume (mL)/BSA1.3 | 168 | 45.3 (35.7,55.7) | 189 | 45.8 (36.2,55.7) | 0.81 |
| End-diastolic area (cm2)/BSA0.8 | 245 | 21.3±4.8 | 258 | 20.7±4.4 | 0.10 |
| Eccentricity index | 245 | 1.34±0.33 | 259 | 1.34±0.35 | 0.95 |
| Degree of tricuspid insufficiency | 260 | 274 | 0.47, 0.51* | ||
| None | 77 (30%) | 92 (34%) | |||
| Mild | 146 (56%) | 154 (56%) | |||
| Moderate | 32 (12%) | 25 (9%) | |||
| Severe | 5 (2%) | 3 (1%) | |||
Data presented as mean ±SD, median (inter-quartile range) or n (%).
P-values obtained from Fisher’s exact test and Mantel-Haenszel test of trend respectively.
MBTS=modified Blalock-Taussig shunt; RVPAS=right ventricular-to-pulmonary artery shunt; RV=right ventricle
Postoperative Assessment
Right Ventricular Systolic, Diastolic and Global Function
RV ejection fraction remained low (mean < 50%) at all post-operative intervals for both groups (Table 3). At the post-Norwood study, the following were significantly higher in the RVPAS group compared to the MBTS group: RV ejection fraction (49±7% vs. 44±8%; P<0.0001); RV % area change (38±7% vs. 35±8%; P<0.001); presence of pulmonary vein reversal of flow with atrial contraction (21% vs. 8%, P<0.001); and myocardial performance index (MPI) based on blood flow Doppler calculation (0.58±0.25 vs. 0.38±0.18; P<0.001). MPI based on blood flow Doppler calculations was also significantly higher in the RVPAS group at the pre-stage II study (0.49±0.20 vs.0.39±0.18; P<0.001), but was not different at any interval between shunt types when MPI was calculated from annular DTI tracings. All other differences became non-significant at the pre-stage II study. No RV functional indices, including MPI by any method, were significantly different at age 14 months.
Table 3.
2D echocardiographic-Doppler indices of RV systolic, diastolic and global function at three post-operative intervals by shunt type
| Variable | Post-Norwood | Pre-stage II | 14 months | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| N | MBTS | N | RVPAS | p | N | MBTS | N | RVPAS | p | N | MBTS | N | RVPAS | p | |
| RV systolic function | |||||||||||||||
| RV ejection fraction % (biplane pyramidal) | 169 | 44±8 | 198 | 49±7 | <0.001 | 127 | 43±8 | 153 | 45±9 | 0.12 | 104 | 43±7 | 132 | 43±8 | 0.91 |
| RV % area change | 224 | 35±8 | 236 | 38±7 | <0.001 | 167 | 33±7 | 208 | 34±8 | 0.45 | 134 | 33±7 | 170 | 32±7 | 0.49 |
| RV dP/dt, mmHg/sec | 46 | 1032±330 | 41 | 983±336 | 0.50 | 35 | 865±301 | 47 | 871±335 | 0.93 | 24 | 798±318 | 28 | 854±402 | 0.59 |
| Tricuspid annular tissue Doppler peak systolic velocity, cm/sec | 205 | 4.51±1.36 | 213 | 4.40±1.11 | 0.38 | 153 | 5.80±1.98 | 188 | 5.22±1.43 | 0.003 | 140 | 5.46±1.49 | 171 | 5.29±1.37 | 0.28 |
| Tricuspid annular tissue Doppler isovolumic acceleration, cm/sec/sec | 41 | 26.6±12.8 | 29 | 26.7±10.9 | 0.97 | 57 | 31.8±11.3 | 69 | 31.3±12.7 | 0.80 | 71 | 35.2±11.7 | 95 | 35.1±11.2 | 0.95 |
| RV diastolic function | |||||||||||||||
| Peak tricuspid inflow E velocity, m/sec | 217 | 0.83±0.26 | 224 | 0.79±0.23 | 0.09 | 160 | 0.86±0.26 | 199 | 0.86±0.25 | 0.87 | 141 | 0.86±0.25 | 179 | 0.86±0.25 | 0.91 |
| Peak tricuspid inflow A velocity, m/sec | 91 | 0.66±0.21 | 108 | 0.66±0.19 | 0.90 | 94 | 0.80±0.21 | 107 | 0.74±0.21 | 0.07 | 80 | 0.72±0.22 | 96 | 0.72±0.20 | 0.97 |
| Tricuspid inflow E/A ratio | 91 | 1.03±0.44 | 108 | 1.01±0.27 | 0.62 | 94 | 0.95±0.25 | 107 | 1.01±0.27 | 0.07 | 80 | 1.12±0.50 | 96 | 1.07±0.26 | 0.34 |
| Tricuspid annular tissue Doppler E′ velocity, cm/sec | 203 | 7.63±2.99 | 212 | 7.61±2.84 | 0.96 | 154 | 8.54±3.59 | 187 | 8.98±3.45 | 0.25 | 142 | 8.45±3.47 | 172 | 8.95±3.87 | 0.23 |
| Tricuspid annular tissue Doppler A′ velocity, cm/sec | 95 | 6.39±2.04 | 115 | 6.87±2.51 | 0.13 | 93 | 7.39±2.5 | 113 | 7.64±2.39 | 0.47 | 75 | 6.85±2.26 | 99 | 6.79±2.24 | 0.88 |
| E/E′ ratio | 196 | 12.2±5.9 | 206 | 11.5±5.4 | 0.26 | 148 | 11.3±4.9 | 181 | 10.2±4.1 | 0.02 | 138 | 11.5±5.1 | 169 | 11.0±4.7 | 0.30 |
| Presence of pulmonary vein flow reversal | 212 | 17 (8%) | 216 | 46 (21%) | <0.001 | 157 | 18 (12%) | 191 | 33 (17%) | 0.13 | 135 | 9 (7%) | 176 | 12 (7%) | 1.00 |
| Myocardial Performance Index or Tei Index | |||||||||||||||
| Blood flow Doppler calculation | 200 | 0.38±0.18 | 198 | 0.58±0.25 | <0.001 | 150 | 0.39±0.18 | 184 | 0.49±0.20 | <0.001 | 131 | 0.44±0.16 | 168 | 0.44±0.18 | 0.96 |
| Tissue Doppler calculation | 83 | 0.65±0.35 | 70 | 0.64±0.25 | 0.79 | 63 | 0.56±0.21 | 83 | 0.56±0.19 | 0.91 | 80 | 0.59±0.21 | 96 | 0.60±0.18 | 0.69 |
Data presented as mean±SD, median (inter-quartile range), or n (%). MBTS=modified Blalock-Taussig shunt; RVPAS=right ventricular-to-pulmonary artery shunt; RV=right ventricle
Cardiac and Vascular Anatomic Dimensions
RV shape as assessed by eccentricity index and RV size by area and volume analysis were not significantly different between shunt groups at any interval (Table 4). Trends toward smaller end-diastolic and end-systolic RV volumes were seen in the RVPAS group at both the post-Norwood and pre-stage II studies, but these differences disappeared at the 14 month visit. Left pulmonary artery diameter (0.39±0.09 vs. 0.42±0.11 cm, P<0.001) and z-score (−1.12±1.07 vs. −0.71±1.26, P<0.001) were smaller in the MBTS group at the post-Norwood assessment, but no other pulmonary artery or neo-aortic anatomic measure was significantly different at any post-operative stage between groups.
Table 4.
2D echocardiographic indices of cardiac and vascular anatomic dimension at three post-operative intervals by shunt type
| Variable | Post-Norwood | Pre-stage II | 14 months | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| N | MBTS | N | RVPAS | p | N | MBTS | N | RVPAS | p | N | MBTS | N | RVPAS | p | |
| RV eccentricity index | 225 | 1.28±0.29 | 236 | 1.27±0.34 | 0.56 | 167 | 1.36±0.33 | 210 | 1.35±0.35 | 0.62 | 134 | 1.30±0.29 | 170 | 1.28±0.31 | 0.53 |
| RV End-diastolic volume (mL)/BSA1.3 | 169 | 89.5 (75.0,106.3) | 198 | 88.5 (74.5,104.8) | 0.66 | 126 | 112.8 (94.0,137.7) | 153 | 105.2 (86.8,124.6) | 0.01 | 104 | 83.6 (70.1,100.4) | 132 | 88.7 (72.4,103.9) | 0.16 |
| RV End-systolic volume (mL)/BSA1.3 | 169 | 49.9 (38.9,61.3) | 198 | 44.7 (37.2,57.8) | 0.01 | 126 | 63.1 (49.8,81.1) | 153 | 58.2 (44.6,70.3) | 0.009 | 104 | 46.3 (38.7,58.0) | 132 | 50.0 (40.0,60.1) | 0.16 |
| RV End-diastolic area (cm2)/BSA0.8 | 224 | 21.9±4.9 | 236 | 22.1±5.0 | 0.63 | 166 | 27.0±6.3 | 208 | 25.6±5.7 | 0.02 | 134 | 22.8±4.5 | 170 | 24.4±5.9 | 0.01 |
| Neo-aortic narrowest distal arch dimension, cm | 215 | 0.64±0.16 | 227 | 0.62±0.15 | 0.14 | 163 | 0.69±0.18 | 180 | 0.63±0.17 | 0.002 | 132 | 0.81±0.21 | 163 | 0.77±0.21 | 0.10 |
| Pulmonary artery size | |||||||||||||||
| LPA diameter, cm | 214 | 0.39±0.09 | 215 | 0.42±0.11 | <0.001 | 142 | 0.45±0.12 | 156 | 0.46±0.11 | 0.31 | 112 | 0.50±0.12 | 129 | 0.48±0.12 | 0.38 |
| LPA z-score | 214 | −1.12±1.07 | 215 | −0.71±1.26 | <0.001 | 142 | −1.46±1.19 | 156 | −1.35±1.18 | 0.41 | 112 | −1.85±1.08 | 129 | −2.01±1.16 | 0.27 |
| Proximal RPA diameter, cm | 196 | 0.41±0.10 | 218 | 0.43±0.10 | 0.07 | 116 | 0.46±0.10 | 169 | 0.45±0.11 | 0.69 | 110 | 0.48±0.11 | 128 | 0.48±0.13 | 0.82 |
| Proximal RPA z-score | 196 | −1.20±1.11 | 218 | −0.99±1.12 | 0.06 | 116 | −1.71±1.05 | 169 | −1.81±1.14 | 0.43 | 110 | −2.43±1.03 | 128 | −2.42±1.24 | 0.96 |
| Distal RPA diameter, cm | --- | --- | --- | --- | --- | --- | --- | --- | --- | --- | 81 | 0.63±0.15 | 89 | 0.61±0.13 | 0.38 |
Data presented as mean±SD, median (inter-quartile range), or n (%). MBTS=modified Blalock-Taussig shunt; RVPAS=right ventricular-to-pulmonary artery shunt; RV=right ventricle; LPA=left pulmonary artery; RPA=right pulmonary artery
Neo-aortic and Tricuspid Annular Dimensions and Valvar Function
Neo-aortic and tricuspid annulus dimensions and areas were significantly greater than normal in both shunt groups with z-scores ranging from 3 to 7 (Table 5). Post-Norwood, neo-aortic valve annulus area (4.21±1.13 vs. 4.64±1.16 cm2/BSA; P<0.001) and anteroposterior diameter (2.26±0.36 vs. 2.38±0.35 cm/BSA; P<0.001) and their corresponding z-scores (Figure 1) were significantly smaller in the RVPAS group compared to the MBTS group. Neo-aortic annulus dimension z-scores remained significantly smaller at pre-stage II in the RVPAS group (P<0.001) but the differences were no longer statistically significant at 14 months. Significant neo-aortic regurgitation, graded as moderate or severe, was uncommon (≥3%) and was not different between shunt types at 14 months. Tricuspid valve size and degree of regurgitation were similar in the two groups at all post-operative intervals, with 20% of the MBTS and 23% of the RVPAS cohort having moderate or severe regurgitation at 14 months.
Table 5.
2D echocardiographic indices of neo-aortic and tricuspid valve dimensions and functions at three post-operative intervals by shunt type.
| Variable | Post-Norwood | Pre-stage II | 14 months | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| N | MBTS | N | RVPAS | p | N | MBTS | N | RVPAS | p | N | MBTS | N | RVPAS | p | |
| Neo-aortic valve | |||||||||||||||
| Neo-aortic valve annular area (cm2)/BSA | 224 | 4.64±1.16 | 228 | 4.21±1.13 | <0.001 | 164 | 4.91±1.21 | 205 | 4.15±1.15 | <0.001 | 137 | 4.31±1.08 | 171 | 4.18±1.09 | 0.33 |
| Neo-aortic valve annular area, z-score | 224 | 7.04±3.02 | 228 | 5.92±2.94 | <0.001 | 164 | 8.02±3.24 | 205 | 5.99±3.09 | <0.001 | 137 | 6.53±2.96 | 171 | 6.20±2.98 | 0.34 |
| Neo-aortic AP valve annulus diameter (cm2)/√BSA | 229 | 2.38±0.35 | 234 | 2.26±0.36 | <0.001 | 168 | 2.44±0.35 | 212 | 2.23±0.33 | <0.001 | 143 | 2.30±0.33 | 176 | 2.26±0.36 | 0.23 |
| Neo-aortic AP valve annulus diameter, z-score | 229 | 4.70±2.02 | 234 | 4.01±2.06 | <0.001 | 168 | 5.60±2.26 | 212 | 4.29±2.09 | <0.001 | 143 | 5.02±2.22 | 176 | 4.72±2.42 | 0.25 |
| Degree of neo-aortic valve regurgitation | 238 | 242 | 0.19, 0.07* | 175 | 220 | 0.17, 0.73* | 147 | 186 | 0.08, 0.08* | ||||||
| none | 132 (56%) | 120 (50%) | 106 (61%) | 148 (67%) | 65 (44%) | 104 (56%) | |||||||||
| mild | 103 (43%) | 114 (47%) | 66 (38%) | 69 (31%) | 77 (52%) | 79 (43%) | |||||||||
| moderate | 3 (1%) | 8 (3%) | 3 (2%) | 1 (1%) | 5 (3%) | 3 (1%) | |||||||||
| severe | 0 (0%) | 0 (0%) | 0 (0%) | 2 (1%) | 0 (0%) | 0 (0%) | |||||||||
| Tricuspid valve | |||||||||||||||
| Tricuspid annular area (cm2)/BSA | 224 | 7.10±2.02 | 232 | 7.01±2.01 | 0.63 | 171 | 7.27±2.20 | 211 | 7.07±2.62 | 0.42 | 146 | 6.18±1.95 | 181 | 6.61±2.09 | 0.05 |
| Tricuspid annular area, z-score | 224 | 3.23±1.88 | 232 | 3.16±1.93 | 0.70 | 171 | 3.82±2.31 | 211 | 3.62±2.77 | 0.46 | 146 | 2.89±2.25 | 181 | 3.41±2.43 | 0.05 |
| Tricuspid AP valve annulus diameter (cm2)/√BSA | 225 | 2.89±0.50 | 235 | 2.86±0.48 | 0.60 | 171 | 2.92±0.50 | 213 | 2.85±0.58 | 0.22 | 148 | 2.65±0.50 | 182 | 2.79±0.51 | 0.02 |
| Tricuspid AP valve annulus diameter,z-score | 225 | 2.12±1.71 | 235 | 2.04±1.63 | 0.63 | 171 | 2.46±1.79 | 213 | 2.22±2.10 | 0.24 | 148 | 1.61±1.86 | 182 | 2.12±1.89 | 0.01 |
| Tricuspid transverse valve annulus diameter (cm2)/√BSA | 235 | 3.07±0.51 | 238 | 3.07±0.51 | 0.97 | 174 | 3.13±0.54 | 220 | 3.07±0.58 | 0.33 | 146 | 2.91±0.51 | 184 | 2.96±0.51 | 0.36 |
| Tricuspid transverse valve annulus diameter, z-score | 235 | 1.78±1.30 | 238 | 1.80±1.35 | 0.91 | 174 | 2.32±1.55 | 220 | 2.17±1.68 | 0.37 | 146 | 1.95±1.61 | 184 | 2.14±1.61 | 0.31 |
| Degree of tricuspid regurgitation | 237 | 243 | 0.68, 0.86* | 175 | 222 | 0.09, 0.63* | 148 | 185 | 0.88, 0.89* | ||||||
| none | 28 (12%) | 29 (12%) | 31 (18%) | 50 (23%) | 24 (16%) | 27 (15%) | |||||||||
| mild | 148 (62%) | 159 (65%) | 97 (55%) | 114 (51%) | 94 (64%) | 115 (62%) | |||||||||
| moderate | 48 (20%) | 47 (19%) | 33 (19%) | 51 (23%) | 25 (17%) | 34 (18%) | |||||||||
| severe | 13 (6%) | 8 (3%) | 14 (8%) | 7 (3%) | 5 (3%) | 9 (5%) | |||||||||
Data presented as mean±SD or n (%). MBTS=modified Blalock-Taussig shunt; RVPAS=right ventricular-to-pulmonary artery shunt
P-values obtained from Fisher’s exact test and Mantel-Haenszel test of trend respectively.
Figure 1.
Boxplots are shown by shunt type at all four intervals for neo-aortic valve annular area z-scores (panel A; pre-Norwood N=473, post-Norwood N=452, pre-stage II N=369, 14 months N=308) and neo-aortic anteroposterior (AP) valve annulus diameter z-scores (panel B; pre-Norwood N=521, post-Norwood N=463, pre-stage II N=380, 14 months N=319). Z-scores for both neo-aortic area and diameter are significantly smaller in the RVPAS group compared compared to MBTS (P<0.001) post-Norwood and pre-stage II, but are not significantly different at baseline and 14 months.
Neo-aortic Flow Patterns
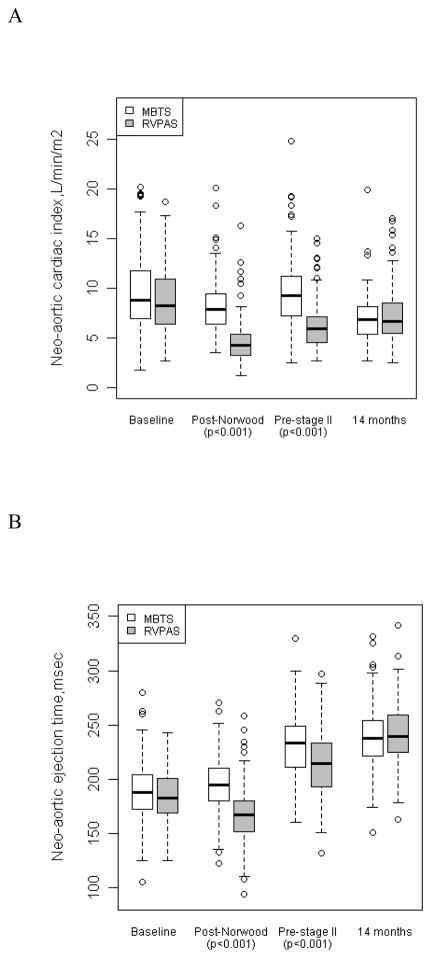
All indices of neo-aortic flow (cardiac index, ejection time, and peak distal arch velocity) were significantly lower in the RVPAS compared to the MBTS group (Figure 2) at both interstage studies (post-Norwood and pre-Stage II; P<0.001). In addition, the percentage of retrograde diastolic flow in the thoracic descending neo-aorta was significantly increased in the MBTS group compared to the RVPAS, both post-Norwood (median [IQR] 43% [33–54%] vs. 0% [0–0%], P<0.001) and pre-Stage II (39% [31–50%] vs. 0% [0–0%], P<0.001). All neo-aortic flow indices were similar for the two shunt types at age 14 months.
Figure 2.
Boxplots are shown by shunt type at all four intervals for neo-aortic cardiac index (panel A; pre-Norwood N=327, post-Norwood N=397, pre-stage II N=330, 14 months N=286), neo-aortic ejection time (panel B; pre-Norwood N=363, post-Norwood N=419, pre-stage II N=353, 14 months N=303), and neo-aortic peak distal arch velocity (panel C; post-Norwood N=355, pre-stage II N=298, 14 months N=264). All 3 neo-aortic flow indices are significantly different (P<0.001) at post-Norwood and pre-stage II with decreased cardiac index, ejection time and peak distal arch velocity in the RVPAS compared to MBTS, but these indices were not significantly different at baseline and 14 months.
Sub-Analysis of Subjects Alive at 14 Months
To investigate the possibility of survivor bias, all 2D/Doppler indices were re-examined in the subgroup of subjects alive at 14 months who had echocardiographic studies deemed acceptable at all 3 post-baseline time points (n=329). The direction of shunt effect was consistent and all indices found significant for the entire cohort remained statistically significant in this subgroup at all intervals (Online Data Supplement Table). Similarly, all non-significant comparisons by shunt type of 2D/Doppler echo indices for the entire cohort remained non-significant in the subgroup of subjects alive at 14 months.
Discussion
This SVR study demonstrates that initial shunt type for the Norwood procedure does not impact echocardiographic indices measured after shunt removal at 14 months of age, including RV systolic, diastolic and global function, cardiac and vascular dimensions, neo-aortic and tricuspid annulus dimensions and valve function, and neo-aortic flow patterns. Previous single-center studies in children with HLHS comparing shunt types have been limited by relatively small sample size with qualitative or incomplete quantitative assessment of RV fractional area change and have presented conflicting results. Hughes10 identified better early post-operative RV systolic function after RVPAS compared to MBTS, while others found either no difference between shunt groups11,12 or decreased RV function early after RVPAS.13 The few studies using more innovative echocardiographic techniques to assess RV function after the Norwood procedure, including dP/dt from the TR jet,14 tricuspid annular DTI,11,15 indices of diastolic function,11 and the MPI11 have also been limited by small sample size at a single center. In contrast, this study is the first multi-center trial specifically designed to compare echocardiographic indices in a large cohort of patients randomized to receive either a RVPAS or MBTS at initial palliation.7 Moreover, comparison of baseline anatomic features and RV functional status of the SVR trial subjects prior to Norwood palliation shows an appropriately randomized cohort with no significant differences between the MBTS and RVPAS groups prior to surgery.
There were relatively few interstage differences in echocardiographic indices between shunt types, and many of these differences could be explained by the different physiologies created by the shunts. Smaller neo-aortic annulus dimensions, shorter neo-aortic systolic ejection times, lower neo-aortic cardiac index, decreased peak aortic arch velocities and increased blood flow-calculated MPI were found in the RVPAS compared to the MBTS. These findings are consistent with diminished flow across the neo-aortic valve and can be explained at least in part by the physiology of the RVPAS. With the RVPAS in place, a portion of the RV output flows directly into the pulmonary bed via the conduit rather than across the neo-aortic valve; this is in contrast to MBTS patients, where all RV output must flow across the neo-aortic valve. A shortened neo-aortic ejection time after Norwood in patients with the RVPAS compared to the MBTS has been previously described11 with early neo-aortic valve closure likely secondary to more rapid systolic pressure decay as the RV is able to eject into both the pulmonary and systemic vascular beds. This difference in flow is expected as part of the unique physiology of the RVPAS rather than a pathologic change reflecting myocardial disease, and this difference likely explains and invalidates the higher interstage MPI values found in the RVPAS cohort using blood flow calculations (because of the shortened neo-aortic ejection time). Indeed, when MPI was calculated from myocardial tissue Doppler indices, instead of blood flow, no significant differences were noted between groups at any stage.
Although the lack of differences in cardiac size and function at 14 months suggests that the impact of the initial shunt physiology appears to diminish over time after shunt removal, the window of observation in this study was short. Previous single-center studies comparing outcome in later childhood between shunt types again provide conflicting results. Despite better early survival with the RVPAS compared to the MBTS, several reports have identified poorer qualitative RV systolic function prior to and after the final stage Fontan palliation in patients who had the RVPAS.16,17 The RVPAS requires a ventriculotomy that could potentially impact ventricular performance because of myocardial injury, scar, or aneurysm formation.18 The increased prevalence of reversal of pulmonary vein flow with atrial contraction and a trend towards smaller ventricular volumes in the RVPAS group at the post-Norwood study may reflect more restrictive diastolic function as a result of the ventriculotomy early after surgery, similar to what has been described in infants early after tetralogy of Fallot repair.19 The impact of shunt type after subsequent palliations remains unclear, however, as a recent report found no shunt-related difference in RV function after Fontan, but worse tricuspid valve function in the MBTS patients.20
In this cohort differential neo-aortic annulus growth related to shunt type diminished at 14 months. Not surprisingly, the neo-aortic annulus remained dramatically dilated in both shunt groups at all stages compared to normal controls. Other studies have reported the development of significant neo-aortic root dilatation and neo-aortic valve insufficiency late after Norwood palliation with a MBTS.21 This study may not have been of sufficient duration to know if this cohort will show the same degree of progressive disease. Similarly, despite the lack of important differences in echo-derived measures of the pulmonary arteries (the only statistically significant difference being the left pulmonary artery size discrepancy of 0.39 vs 0.42 cm post-Norwood) and distal aortic arch size at 14 months in this cohort, the impact of shunt type on late vascular growth remains unclear.
This cohort provides a unique opportunity for longitudinal follow-up, and continued monitoring of SVR trial survivors is already in place. A Pediatric Heart Network-sponsored SVR extension study is ongoing, designed to provide additional clinical and echocardiographic surveillance through age 6 years, with protocol echocardiograms obtained within 6 months of Fontan palliation and at 6 years of age. This extension should help identify longer term effects of initial shunt type on echocardiographic indices of cardiac, valvar, and vascular size and function as this cohort progresses through Fontan palliation, and it is anticipated that these children will continue to be monitored into adolescence and adulthood.
Study Limitations
Unlike for the left ventricle, 2D echocardiographic tools for assessment of RV volume and systolic functional assessment remain limited secondary to the complex geometry of the chamber. The biplane pyramidal method for RV size assessment used here (Online Data Supplement) has been shown to correlate well with RV ejection fraction estimates derived from cardiac magnetic resonance imaging in children with and without congenital heart disease, and it appears to provide the most accurate estimate of RV volume when compared to other described 2D echo RV volume methods.6 Of note, analysis of regional RV wall motion was not performed as part of this protocol, and so the impact of focal scarring/dyskinesis was not specifically assessed.
Careful training in protocol image acquisition was provided and reinforced at all sites with regular quality assurance feedback from the core lab to optimize appropriate image capture, and 99% of the submitted studies were found to be acceptable in providing images that allowed data extraction. However, more innovative measurements such as RV dP/dt and tricuspid annular isovolumic acceleration were not possible in the majority of studies because of incomplete or inadequate image acquisition. The impact of this limitation in the characterization of RV function for the SVR cohort is unclear but emphasizes the challenge of multi-institutional echocardiographic trials that require extensive data acquisition, particularly when obtaining those data from infants with complex heart disease.
Conclusions
Indices of RV function, cardiac and vascular dimensions, valve annulus dimensions and function, and neo-aortic flow patterns in survivors of the Norwood procedure are similar for subjects with MBTS and RVPAS by 14 months of age. Interstage differences in neo-aortic annular size and flow patterns between shunt types can likely be explained by the different physiologies created when the shunts are in place rather than by intrinsic differences in myocardial and valve function. Longitudinal follow-up of the cohort is ongoing and will likely provide further insights into the long-term effect of initial shunt type on clinical outcome and echocardiographic indices of cardiac size and function.
Supplementary Material
Acknowledgments
Funding Sources
Supported by U01 grants from the National Heart, Lung, and Blood Institute (HL068269, HL068270, HL068279, HL068281, HL068285, HL068292, HL068290, HL068288, HL085057). The contents of this work are solely the responsibility of the authors and do not necessarily represent the official views of the National Heart, Lung, and Blood Institute
Footnotes
Disclosures: none
Contributor Information
Peter C Frommelt, Medical College of Wisconsin, Milwaukee, WI.
Lin T Guey, New England Research Institute, Watertown, MA.
L LuAnn Minich, University of Utah, Salt Lake City, UT.
Majeed Bhat, Nemours Cardiac Center, Wilmington, DE.
Tim J Bradley, Hospital for Sick Children, Toronto, ON, Canada.
Steve D Colan, Children’s Hospital Boston, Boston, MA.
Greg Ensing, University of Michigan, Ann Arbor, MI.
Jessica Gorentz, Medical College of Wisconsin, Milwaukee, WI.
Haleh Heydarian, Cincinnati Children’s Hospital, Cincinnati, OH.
J Blaine John, Pediatric Cardiology Associates/Pediatrix, Tampa, FL.
Wyman W Lai, Columbia University, New York, NY.
Jami C. Levine, Children’s Hospital Boston, Boston, MA.
William T Mahle, Emory University, Atlanta, GA.
Stephen G. Miller, Duke University, Durham, NC.
Richard G Ohye, University of Michigan, Ann Arbor, MI.
Gail D Pearson, National Heart, Lung, and Blood Institute, Bethesda, MD.
Girish S Shirali, Medical University of South Carolina, Charleston, SC.
Pierre C. Wong, Children’s Hospital Los Angeles, Los Angeles, CA.
Meryl S Cohen, The Children’s Hospital of Philadelphia, Philadelphia, PA.
References
- 1.Norwood WI, Lang P, Hansen DD. Physiologic repair of aortic atresia-hypoplastic left heart syndrome. N Engl J Med. 1983;308:23–6. doi: 10.1056/NEJM198301063080106. [DOI] [PubMed] [Google Scholar]
- 2.Sano S, Ishino K, Kawada M, Arai S, Kasahara S, Asai T, Masuda Z, Takeuchi M, Ohtsuke S. Right ventricle-pulmonary artery shunt in first-stage palliation of hypoplastic left heart syndrome. J Thorac Cardiovasc Surg. 2003;126:504–10. doi: 10.1016/s0022-5223(02)73575-7. [DOI] [PubMed] [Google Scholar]
- 3.Alsoufi B, Bennetts J, Verma S, Caldarone CA. New Developments in the Treatment of Hypoplastic Left Heart Syndrome. Pediatrics. 2007;119:109–117. doi: 10.1542/peds.2006-1592. [DOI] [PubMed] [Google Scholar]
- 4.Mahony LLA, Sleeper LA, Anderson PA, Gersony WM, McCrindle BW, Minich LL, Newburger JW, Saul JP, Vetter VL, Pearson GD. The Pediatric Heart Network: a primer for the conduct of multicenter studies in children with congenital and acquired heart disease. Pediatr Cardiol. 2006;27:191–198. doi: 10.1007/s00246-005-1151-9. [DOI] [PubMed] [Google Scholar]
- 5.Ohye RG, Sleeper LA, Mahony L Newburger JW, Pearson GD, Lu M, Goldberg CS, Tabbutt S, Frommelt PC, Ghanayem NS, Laussen PC, Rhodes JF, Lewis AB, Mital S, Ravishankar C, Williams IA, Dunbar-Masterson C, Atz AM, Colan S, Minich LL, Pizarro C, Kanter KR, Jaggers J, Jacobs JP, Krawczeski CD, Pike N, McCrindle BW, Virzi L, Gaynor JW Pediatric Heart Network Investigators. Comparison of shunt types in the Norwood procedure for single-ventricle lesions. N Engl J Med. 2010;362(21):1980–92. doi: 10.1056/NEJMoa0912461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Helbing WA, Bosch HG, Maliepaard C, Rebergen SA, van der Geest RJ, Hansen B, Ottenkamp J, Reiber JHC, de Roos A. Comparison of echocardiographic methods with magnetic resonance imaging for assessment of right ventricular function in children. Am J Cardiol. 1995;76:589–594. doi: 10.1016/s0002-9149(99)80161-1. [DOI] [PubMed] [Google Scholar]
- 7.Ohye RG, Gaynor JW, Ghanayem NS, Goldberg CS, MD, Laussen PC, Frommelt PC, Newburger JW, Pearson GD, MD, Tabbutt S, MD, Wernovsky G, Wruck LM, Atz AM, Colan SD, MD, Jaggers J, McCrindle BW, Prakash A, MD, Puchalski MJ, Sleeper LA, Stylianou MP, Mahony M for the Pediatric Heart Network Investigators. Design and rationale of a randomized trial comparing the Blalock-Taussig and right ventricle-pulmonary artery shunts in the Norwood procedure. J Thorac Cardiovasc Surg. 2003;136:968–975. doi: 10.1016/j.jtcvs.2008.01.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hourihan M, Colan SD, Wernovsky G, Maheswari U, Mayer JE, Sanders S. Growth of the aortic anastomosis, annulus, and root after the arterial switch procedure performed in infancy. Circulation. 1993;88(2):615–20. doi: 10.1161/01.cir.88.2.615. [DOI] [PubMed] [Google Scholar]
- 9.Sluysmans T, Colan SD. Theoretical and empirical derivation of cardiovascular allometric relationships in children. J Appl Physiol. 2005;99(2):445–57. doi: 10.1152/japplphysiol.01144.2004. [DOI] [PubMed] [Google Scholar]
- 10.Hughes ML, Sherkerdemian LS, Brizard CP, Penny DJ. Improved early ventricular performance with a right ventricle to pulmonary artery conduit in stage 1 palliation of hypoplastic left heart syndrome: evidence from strain Doppler echocardiography. Heart. 2004;90:191–194. doi: 10.1136/hrt.2003.016675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Frommelt PC, Sheridan DC, Mussatto KA, Hoffman GM, Ghanayem NS, Frommelt MA, Tweddell JS. Effect of shunt type on echo indices after initial palliations for hypoplastic left heart syndrome: BT shunt vs. RV-PA conduit. J Am Soc Echocardiogr. 2007;20:1364–73. doi: 10.1016/j.echo.2007.04.009. [DOI] [PubMed] [Google Scholar]
- 12.Graham EM, Atz AM, Bradley SM, Scheurer MA, Bandisode VM, Laudito A, Shirali GS. Does a ventriculotomy have deleterious effects following palliation in the Norwood procedure using a shunt placed from the right ventricle to the pulmonary arteries? Cardiol Young. 2007;17:145–150. doi: 10.1017/S1047951107000133. [DOI] [PubMed] [Google Scholar]
- 13.Ballweg JA, Dominguez TE, Ravishankar C, Kreutzer J, Marino BS, Bird GL, Gruber PJ, Wernovsky G, Gaynor JW, Nicolson SC, Spray TL, Tabbutt S. A contemporary comparison of the effect of shunt type in hypoplastic left heart syndrome on the hemodynamics and outcome at stage 2 reconstruction. J ThoracCardiovasc Surg. 2007;134:297–303. doi: 10.1016/j.jtcvs.2007.02.046. [DOI] [PubMed] [Google Scholar]
- 14.Michelfelder EC, Vermilion RP, Ludomirsky A, Beekman RH, Lloyd TR. Comparison of simultaneous Doppler- and catheter-derived right ventricular dP/dt in hypoplastic left heartsyndrome. Am J Cardiol. 1996;77(2):212–214. doi: 10.1016/s0002-9149(96)90604-9. [DOI] [PubMed] [Google Scholar]
- 15.Christensen D, Cardis B, Mahle W, Lewis R, Huckaby J, Favaloro-Sabatier M, Fyfe D. Pre- and postoperative quantitation of right ventricular tissue Doppler velocities in infants with hypoplastic left heart syndrome. Echocardiography. 2006;23:303–307. doi: 10.1111/j.1540-8175.2006.00207.x. [DOI] [PubMed] [Google Scholar]
- 16.Padalino MA, Castellani C, Toffoli S, Della Barbera M, Milanesi O, Thiene G, Stellin G, Angelini A. Pathological changes and myocardial remodeling related to the mode of shunting following surgical palliation for hypoplastic left heart syndrome. Cardiol Young. 2008;18:415–22. doi: 10.1017/S1047951108002461. [DOI] [PubMed] [Google Scholar]
- 17.Ballweg JA, Dominguez TE, Ravishankar C, Gaynor JW, Nicolson SC, Spray TL, Tabbutt S. A contemporary comparison of the effect of shunt type in hypoplastic left heart syndrome on the hemodynamics and outcome at Fontan completion. J Thorac Cardiovasc Surg. 2010;140:537–544. doi: 10.1016/j.jtcvs.2010.03.045. [DOI] [PubMed] [Google Scholar]
- 18.Graham EM, Zyblewski SC, Phillips JW, Shirali GS, Bradley SM, Forbus GA, Bandisode VM, Atz AM. Comparison of Norwood shunt types: do the outcomes differ 6 years later? Ann Thorac Surg. 2010;90:31–35. doi: 10.1016/j.athoracsur.2010.03.078. [DOI] [PubMed] [Google Scholar]
- 19.Cullen S, Shore D, Redington A. Characterization of right ventricular diastolic performance after complete repair of tetralogy of Fallot: restrictive physiology predicts slow postoperative recovery. Circulation. 1995;91:1782–89. doi: 10.1161/01.cir.91.6.1782. [DOI] [PubMed] [Google Scholar]
- 20.Bautista-Hernandez V, Scheurer M, Thiagarajan R, Salvin J, Pigula FA, Emani S, Fynn-Thompson F, Loyola H, Schiff J, del Nido PJ. Right ventricle and tricuspid valve function at midterm after the Fontan operation for hypoplastic left heart syndrome: Impact of shunt type. Pediatr Cardiol. 2011;32:160–66. doi: 10.1007/s00246-010-9835-1. [DOI] [PubMed] [Google Scholar]
- 21.Cohen MS, Marino BS, McElhinney DB, Robbers-Visser D, van der Woerd W, Gaynor JW, Spray TL, Wernovsky G. Neo-aortic root dilatation and valve regurgitation up to 21 years after staged reconstruction for hypoplastic left heart syndrome. J Am Coll Cardiol. 2003;42:533–40. doi: 10.1016/s0735-1097(03)00715-0. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.