Figure 3.
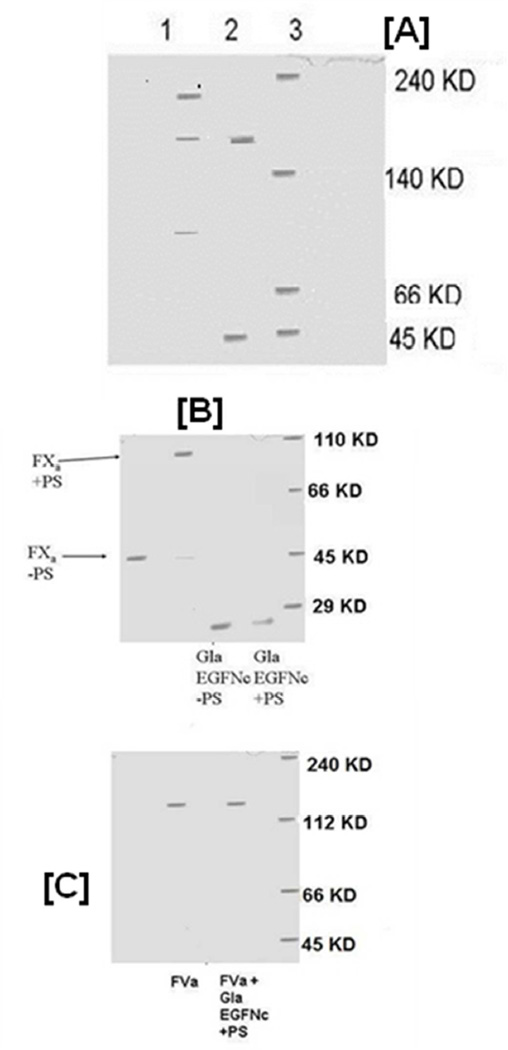
3A. Native Gel Electrophoresis of a FXa and FVa Mixture in the Presence and Absence of C6PS. A 5% polyacrylamide gel of a mixture 50 nM FXa and 50 nM FVa in the presence of 400 µM C6PS and 5 mM Ca2+ (lane 1) shows coexistence of homodimers (FXa ·FXa; 90 KD), heterodimers (FXa · FVa; 224 KD), and FVa (178 KD), showing that FXa monomer interacts with both FVa and with FXa, thus consistent with the hypothesis that FXa and FVa compete to bind FXa. Lane 2 shows that the same reaction mixture in the absence of C6PS contains only monomers of both FVa and FXa, showing that this competition is regulated by phosphatidylserine (in this case C6PS). Lane 3 shows molecular mass markers.
3B: Native Gel Electrophoresis of FXa and its GLA-EGFNC Fragment. 8% native polyacrylamide gel electrophoresis of FXa and GLA-EGFNC fragment of FXa in the presence and absence of µM 400 C6PS and 5 mM Ca2+ shows that the GLA-EGFNC fragment does not dimerize in the presence of C6PS and 5 mM Ca2+. Figure 3C: 5% native polyacrylamide gel electrophoresis of 50 nM FVa with and without 50 nM GLA-αEGFNC domain in the presence of 400 µM C6PS, 5 mM Ca2+. The results show that the GLA-EGFNC fragment of FXa does not form a complex with FVa.
