Figure 7. .
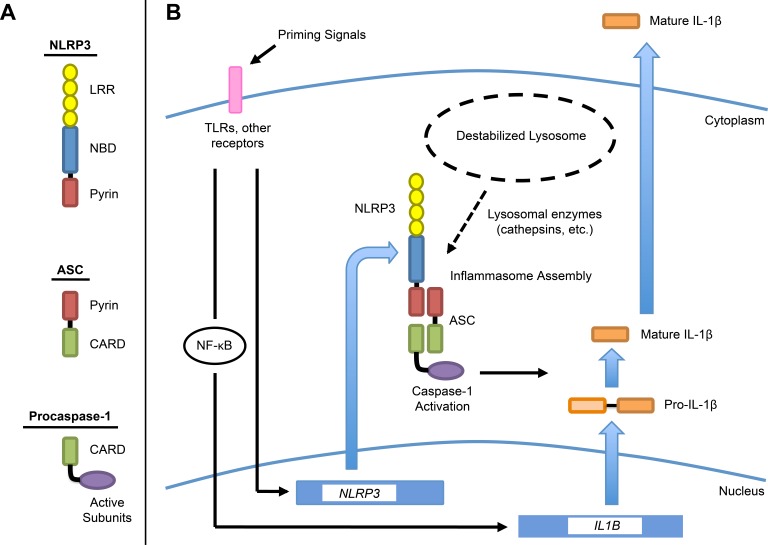
Schematic diagram representing proposed model of NLRP3 inflammasome activation in RPE cells by lysosomal destabilization. (A) Domain architecture of the components of the NLRP3 inflammasome. Inflammasome assembly is mediated by homotypic interactions between pyrin domains on NLRP3 and ASC, and between caspase-recruitment domains (CARDs) on ASC and caspase-1. (B) Two-signal model in which priming signals (signal 1) induce expression of NLRP3 and pro-IL-1β, with pro-IL-1β upregulated via NF-κB. Lysosomal destabilization (signal 2) causes leakage of lysosomal enzymes into the cytosol. These enzymes, such as cathepsins B and L, mediate NLRP3 inflammasome assembly, resulting in caspase-1 activation. Caspase-1 processes pro-IL-1β into mature IL-1β, which is then secreted, and also drives pyroptosis. LRR, leucine-rich repeat; NBD, nucleotide-binding domain.
