Fig. 4. PKCδ regulates NADPH oxidase activity by phosphorylating p47phox and inducing its translocation to the membrane fraction.
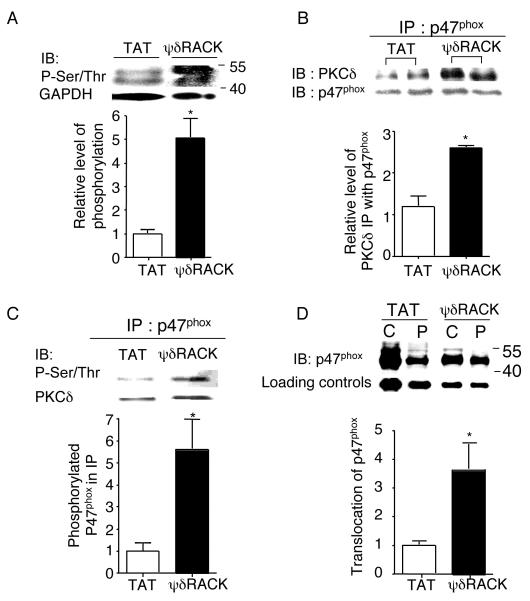
(A) Western blot analyses were performed with total cell lysates of PC-3 tumors treated with TAT or 38 mg/kg/day of ψδRACK for 4 weeks and immunoblotted with antibodies against phospho-serine/threonine (Ser/Thr) (*; p<0.05, n=3 each, Figure 4A). GADPH was used as a loading control. (B) Tumor lysates were immunoprecipitated with antibodies against p47phox and probed for PKCδ. (*; p<0.05, n=3 each, Figure 4B). p47phox was used as a loading control. (C) Phosphorylation levels of the co-immunoprecipitated p47phox were checked by probing with anti-Ser/Thr antibodies (*; p<0.05, n=3 each, Figure 4C). PKCδ was used as a loading control. (D) Finally, tumor lysates were fractionated (as described in the methods) and the translocation of p47phox from the cytosolic to the membrane (particulate) fraction was determined by probing each fraction with anti-p47phox antibodies (Figure 4D, *; p<0.05, n=3 each). GADPH or Gαi was used as a loading control (IB: immunoblot).
