Figure 3. Histology, immunohistochemistry and in situ hybridization of the chick embryos.
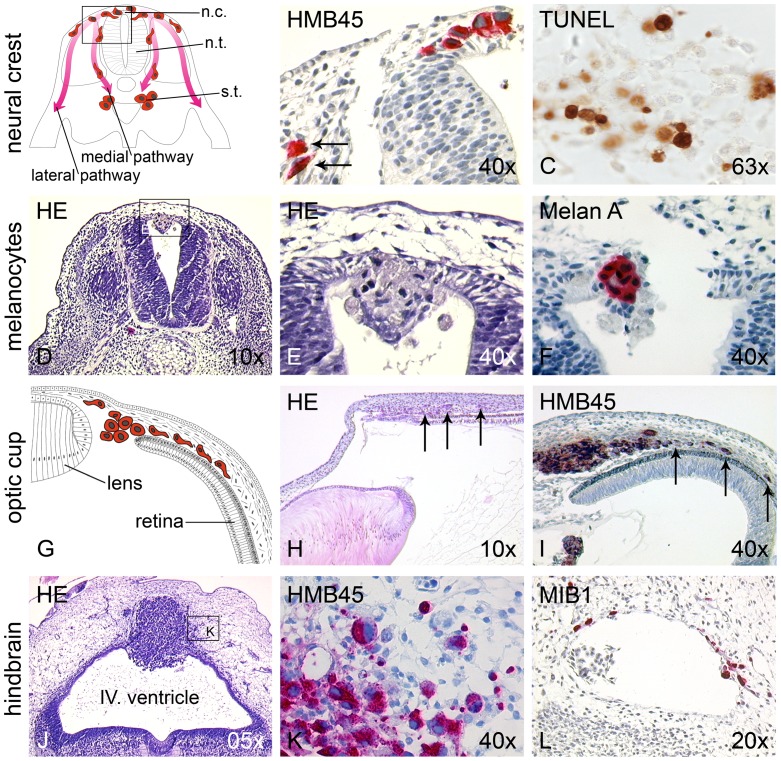
(A) Schematic drawing depicting ventral and medial neural crest migration pathways. n.c. neural crest; n.t. neural tube; s.t. sympathetic trunk. (B) Chick embryo 24 h after transplantation of SKMel28 melanoma cells into the neural tube. Melanoma cells (visualized by HMB45 immunoreactivity) spontaneously resuming neural crest migration have a stretched, mesenchymal-like morphology (arrows). (C) At the site of destination along the ventral migration pathway (para-aortic sympathetic ganglia) melanoma cells undergo apoptosis, visualized by TUNEL staining. (D,E) Chick embryo 24 h after transplantation of benign primary human melanocytes into the neural tube. Melanocytes (showing a compact, epithelial-like morphology) are encountered only in the lumen of the neural tube and, in part, integrated into the roof plate with no neural crest migration. (F) Melan A immunoreactivity confirms the melanocytic origin of the cells. (G) Schematic drawing of chick embryo 72 h after transplantation of B16-F1 melanoma cells into the optic cup. (H) Histological correlate of schematic drawing. Already in H&E staining the transplanted, invasively migrating melanoma cells are visible (arrows). (I) Single melanoma cells (identified by HMB45 immunoreactivity) form a tumor, and single melanoma cells invade the choroid of the optic cup (arrows). (J) Chick embryo 96 h after transplantation of human metastatic melanoma cells into the brain vesicle at the hindbrain (rhombencephalon). The cells form a large tumor in the dorsal neuroepithelium with (K) single HMB45 positive cells infiltrating the surrounding brain tissues. (L) MIB1 immunohistochemistry (proliferation marker not cross-reacting with chick cells) identifies melanoma cells during haematogenous spreading in blood vessels among host erythrocytes and lymphocytes, and in the surrounding neural tissue.
