Figure 3.
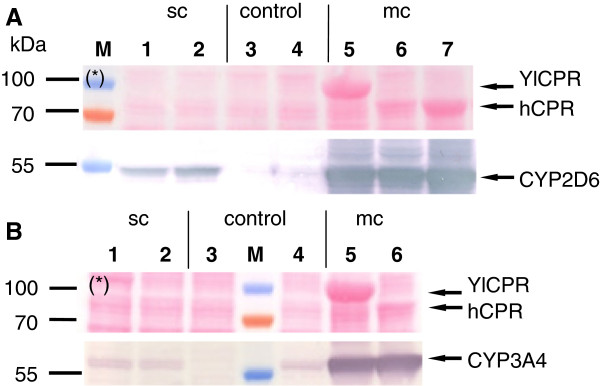
Western blot analysis. Western blot analysis of microsomes isolated after 26 h of ethanol induction from cultures of Y. lipolytica H222-S4 transformed with p64D- or p65D-based integrative vectors, containing different combinations of CYPs and CPRs. Protein transfer was monitored by Ponceau S (*) staining, where putative CPR-protein bands in multicopy transformants were already visible. CYP-protein bands CYP2D6 (A) and CYP3A4 (B) were immunodetected with anti CYP2D6 and CYP3A4 antibodies, respectively.– CYP2D6 was detected in single copy clones (sc) YL12 and YL15 expressing YlCPR-WT + 2D6syn (1), hCPR-WT + 2D6syn (2) respectively and multicopy clones (mc) YL11, YL10 and YL21 expressing YlCPR-WT + 2D6syn (5), hCPRsyn + 2D6syn (6), hCPR-WT + 2D6syn (7) respectively. CYP3A4 was detected in single copy clones (sc) YL19 and YL20 expressing YlCPR-WT + 3A4syn (1), hCPR-WT + 3A4syn (2) respectively and multicopy clones (mc) YL18 and YL22 expressing YlCPR-WT + 3A4syn (5), hCPR-WT + 3A4syn (6) respectively. YL23 clone harboring empty p65D-linker vector (3 + 4 in both panels) was used as negative control. PageRuler Prestained Protein Ladder (M) was used for monitoring protein separation and transfer efficiency.
