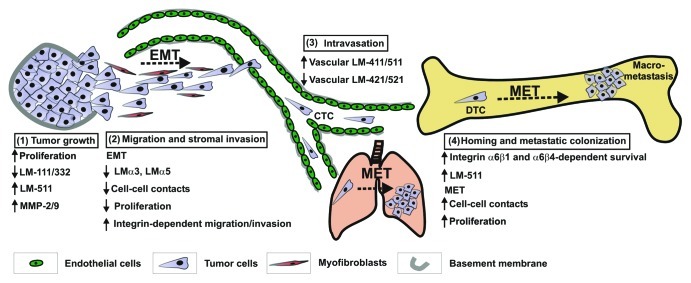
Figure 3. Proposed model of LM-511 expression and function during cancer progression and metastasis. The model depicted above is based primarily on observations made in breast cancer metastasis models but incorporates also findings from studies in other tumor types as referenced below and in the text. (1) Uncontrolled growth of the primary tumor and the loss of myoepithelial-derived LM-111 and LM-332 contribute to the initial disruption of tissue organization.36,41,44 Sustained expression of LM-511 further enhances the breakdown of the surrounding basement membrane through induction of MMP-2/9 gelatinases73,90 resulting in exposure of tumor cells to the surrounding stroma. (2) Under the influence of stromal factors, tumor cells undergo EMT and loss of cell-cell contacts. Snail-dependent downregulation of LMα3 and LMα578,79 may be required to reduce the strength of adhesive interactions and enhance α3β1, α6β1 or α6β4 integrin-dependent cell motility. Subsequent stromal invasion may be achieved by interaction of tumor cells with low level of tumor-derived LM-511 and/or LM-332 derived from myofibrobalsts present at the tumor-stromal interface.49 (3) Changes in the composition of LM isoforms in the tumor vasculature and the abundance of LM-511 are likely to contribute to tumor cell attachment and intravasation.24,29,30 (4) Homing of circulating tumor cells (CTC) and invasion into metastatic sites is mediated through α3β1 integrin interaction with vascular LMs (including LM-511) and MMP-9 proteolytic activity.64,73,91 Binding of integrin α6β1 or α6β4 to endogenous LM-332 or LM-511 (in organs rich in these isoforms) promotes survival and growth of disseminated tumor cells (DTC).62,68,69 Alternatively, enhanced expression of LM-511 and autocrine stimulation may be required in bone where LM-332 or LM-511 expression is more restricted29 to promote strong adhesion and α6β1/α6β4-dependent tumor cell survival. In addition, adhesion to LM-511 may provide the plasticity required for the process of MET and the acquisition of a proliferative epithelial phenotype at metastatic sites.
