Figure 4. Antigenicity of HBeAg and HBcAg.
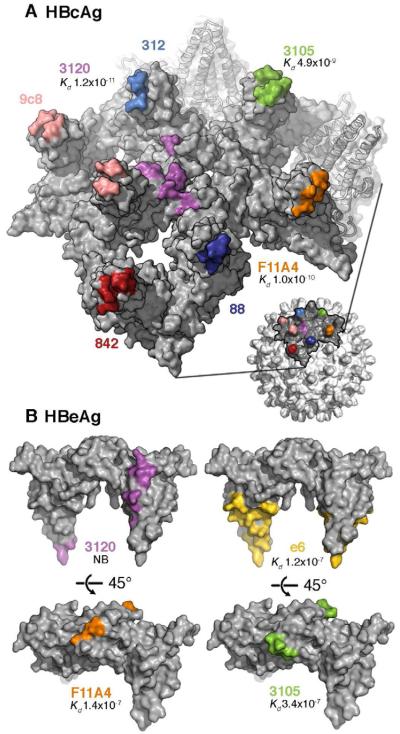
(A) Partial surface of an HBcAg capsid with the epitopes for several anti-HBc/eAg antibodies mapped in colors. Most of these epitopes reside around the spike tips, either on one subunit of the dimeric spike or bridging both subunits. Mab 3120 binds to the floor around the 3-fold and 5-fold symmetry axes.
(B) The same epitopes (matched colors) mapped on the HBeAg dimer. Affinity constants determined by surface plasmon resonance (Kd values (Watts et al., 2010)) are given, where available. A 2 - 3 order of magnitude drop is observed for Mabs F11A4 and 3105 on HBeAg relative to HBcAg, consistent with the constituent loops, which are close together on HBcAg, being well separated on HBeAg.
