Figure 5. Antigenic switching of HBV capsid protein.
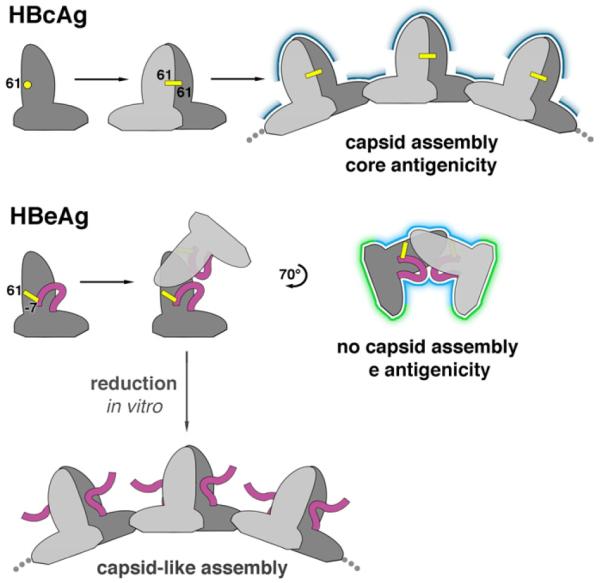
HBcAg and HBeAg polypeptides share the same monomer fold. When HBcAg dimerizes, an inter-molecular disulfide bridge forms between C61 of each subunit. In the HBeAg dimer, however, two intra-molecular disulfides form between C61 and propeptide residue C(-7). Once locked into place, the propeptides block HBcAg dimer formation. Instead, the HBeAg subunits dimerize in an entirely different quaternary arrangement. As a result, HBeAg dimers cannot form the dimer-dimer contacts employed in capsid assembly. The molecular switch also explains how HBeAg and HBcAg are antigenically distinct: surfaces presenting conformational epitopes on HBcAg (dark blue) are altered in HBeAg (light blue) and antibody-inaccessible surfaces on the interior of capsids are exposed in HBeAg (green). Antibodies that bind to only one subunit within the shared surfaces (light and dark blue) may be cross-reactive for both antigens. If HBeAg is subjected to reducing conditions in vitro, these disulfides are disrupted and capsid-like assemblies can form.
