Fig. 3.
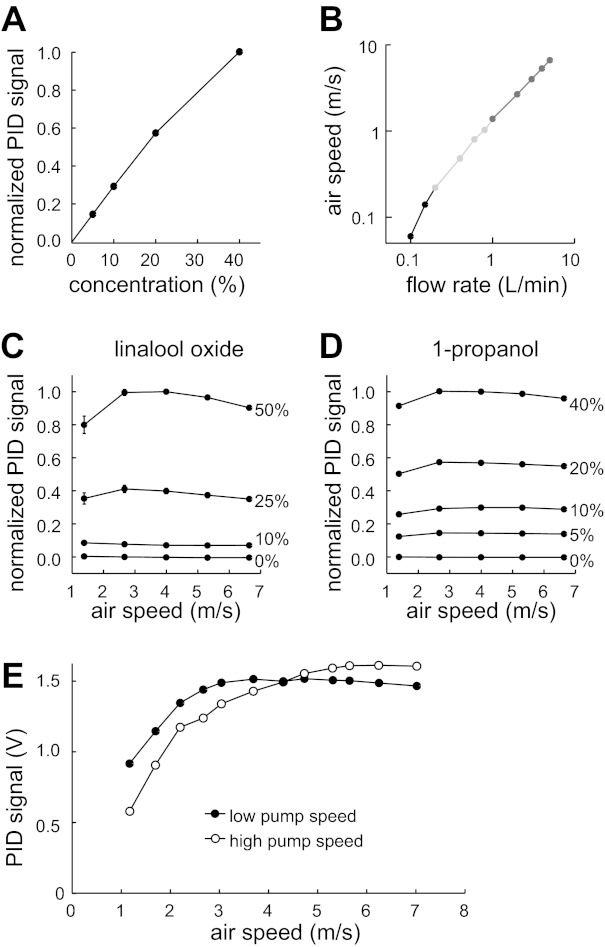
Validation of odor delivery device with PID measurements. A: the normalized PID voltage (which should be proportional to odor concentration at the device outlet) depended nearly linearly on the duty cycle of valve switching (which should be proportional to the concentration in the mixing chamber, reported here as % of saturated vapor). Data were averaged over 11 experiments using linalool oxide at an air speed of 5.3 m/s. B: air speed (as measured by the anemometer) depended linearly on the nominal flow rates delivered through the device. Note log-log axes. Data were collected with 3 different sets of matched flowmeters, labeled here in different shades of gray (see materials and methods). Odor is linalool oxide. C: concentration of linalool oxide delivered to the PID was independent of air speed (mean ± SE, n = 11; some error bars are obscured by markers). D: concentration of 1-propanol delivered to the PID was independent of air speed (mean ± SE; n = 20). E: fall-off in PID signals at low air speeds is more pronounced when the PID pump speed is high (i.e., when the PID is exerting a large negative pressure). This implies that the fall-off is an artifact of the fact that when the PID pump is not completely matched by the odor delivery device outflow, the PID will draw in clean air. Low pump speed is 970 ml/min; high pump speed is 1,652 ml/min. Odor is linalool oxide.
