Abstract
Spleen Tyrosine Kinase (Syk) has been implicated in a number of pathologies including cancer and rheumatoid arthritis and thus has been pursued as a novel therapeutic target. Because of the complex relationship between Syk’s auto- and other internal phosphorylation sites, scaffolding proteins, enzymatic activation state and sites of phosphorylation on its known substrates, the role of Syk’s activity in these diseases has not been completely clear. To approach such analyses, we developed a Syk-specific artificial peptide biosensor (SAStide) to use in a cell-based assay for direct detection of intracellular Syk activity and inhibition in response to physiologically relevant stimuli in both laboratory cell lines and primary splenic B cells. This peptide contains a sequence derived from known Syk substrate preference motifs linked to a cell permeable peptide, resulting in a biosensor that is phosphorylated in live cells in a Syk-dependent manner, thus serving as a reporter of Syk catalytic activity in intact cells. Because the assay is compatible with live, primary cells and can report pharmacodynamics for drug action on an intended target, this methodology could be used to facilitate a better understanding of Syk’s function and the effect of its inhibition in disease.
Syk is a 72 kDa non-receptor tyrosine kinase originally isolated from bovine thymus and porcine spleen1 best known for its role in B lymphocyte development and activation. Loss of Syk expression results in perinatal lethality in mice and an arrest in the development of B cells at the pro-B to pre-B cell and immature to mature B cell transitions.2 Upon antigen binding to the B cell antigen receptor (BCR), the Src family kinase, Lyn initiates the phosphorylation of immunoreceptor tyrosine-based activation motifs (ITAMs) on components of the BCR. Phosphorylation of the ITAMs in turn recruits and activates Syk, inducing its phosphorylation on multiple tyrosines including Y525 and Y526 in its activation loop. Following this activation of Syk, numerous signaling pathways are initiated leading to the activation of downstream transcription factors including NFAT, NFκB and Elk1 and ultimately contributing to the induction of cell proliferation and differentiation.
Dysregulation of the expression or the activity of Syk contributes to various disease states, making it a potential therapeutic target.3 Syk has been implicated as a factor in rheumatological disorders (such as rheumatoid arthritis) and malignant diseases of myeloid, lymphocytic and even epithelial origin. For instance, Syk was found to be constitutively active in primary blasts from a set of patients with acute myeloid leukemia (AML).4 Inhibition of Syk decreased the viability of these AML blasts in vitro and reduced the number of these cells infiltrating spleen and bone marrow in a mouse xenograft model. In some chronic lymphocytic leukemia cells (B-CLL), Syk is hyperactive despite exhibiting normal expression levels,5 and inhibition of Syk or silencing of Syk expression via siRNA decreases cell viability.6 In another example, several peripheral T-cell lymphomas (PTCLs) exhibit aberrant expression of Syk.7 In these cells, siRNA silencing of Syk translation or inhibition of its activity with a kinase inhibitor (R406, Rigel Pharmaceuticals) induces apoptosis and blocks proliferation in cells with elevated Syk Y525/Y526 phosphorylation.8 These results suggest that Syk could be a novel therapeutic target for the treatment of PTCLs. Conversely, in breast cancer, which has an epithelial origin, Syk appears to have tumor suppressor functions: while Syk is expressed in normal breast epithelia, there is little to no Syk present in more metastatic breast cancer cells.9 Expression of Syk negatively affects motility and invasion in these carcinomas.9–10 Accordingly, to guide both the treatment of these cancers and the development of novel therapeutics, it would be beneficial to selectively measure Syk activity in patient samples.
There are three predominant methods currently in use to measure Syk activity: in vitro kinase assays, luciferase reporter assays of downstream transcription factors and phosphotyrosine antibody-based detection of Syk autophosphorylation or substrate sites. Each of these methods has drawbacks that make them less than optimal for both determination of Syk biology and translation to the clinical setting. In vitro kinase assays measure Syk activity post lysis—however, Syk’s function is integrally dependent on its binding partners. For example, in lysed cells proteins such as c-Cbl that normally modulate the function of the kinase (and which are known to be critical for obtaining biologically-relevant activation for Syk) can become separated from Syk; also, proteins normally found in different subcellular compartments are able to artifactually interact with Syk and alter its activity.11 Moreover, as a result of changes in Syk’s phosphorylation state via phosphatase activity and autophosphorylation in vitro, Syk’s activity can change during in vitro kinase assays in ways that may not be relevant to its intracellular activity in a disease context. Transcription factor-driven luciferase reporter assays (another common read-out used for determining Syk activity) are performed in whole cells and maintain the endogenous context of Syk—however, these represent an indirect measure of Syk activity, with numerous proteins in the pathways between Syk and the transcription factors allowing potential disruption of additional components of these cascades. Phosphotyrosine antibody-based methods such as Western blots and Phosphoflow cytometry12 use phosphorylation sites in endogenous proteins, such as known Syk-targeted sites and/or Syk autophosphorylation sites, as surrogate reporters of Syk activity. However, because of the complex relationship between Lyn and Syk activation during B-cell receptor signaling, phosphorylation at endogenous sites (on Syk and other proteins) has not been confirmed to be Syk-specific in all cases. Furthermore, Syk is phosphorylated on multiple sites (by itself and other kinases including Lyn) including some that negatively regulate the kinase,11a making it difficult to parse the complex relationship between the phosphorylation state of these native sites and the intrinsic activation of the enzyme. Besides these functional caveats, the fundamental limitation exists that antibodies for every potentially meaningful site of Syk phosphorylation are not available, and their development is subject to the uncertainties inherent in epitope and antibody generation. Therefore, an ideal method to measure Syk activity would be one that specifically monitors the ability of the kinase to catalyze a phosphotransferase reaction in an intact cell.
Peptide-based approaches offer an alternative method that allows for direct detection of kinase activity. Several peptide substrates for Syk have been previously reported, including some based on endogenous protein sequences and others derived artificially from peptide libraries (through e.g. phage display).13 Peptides have been applied to the detection of Syk activity in vitro using constrained tyrosine analogs and artificial peptides containing fluorescent amino acids.13b, c, 14 Using these substrates, Syk activity has been detected in stimulated cell lysates and from recombinant protein, but so far they have not been used in live, intact cells that provide the necessary context for Syk function. We were interested in developing a peptide biosensor substrate for Syk that could be implemented to detect kinase activity in intact cells using a cell-penetrating peptide approach. TAT-labeled peptide substrates have been used for specific and sensitive detection of endogenous Akt activity in live single cells using capillary electrophoresis.15 Our previous work applied a similar approach to detect intracellular Abl activity in an over-expression model using a peptide biosensor and matrix-assisted laser desorption/absorption ionization-time of flight mass spectrometry (MALDI-TOF) or Western blotting.16 Here we present the development of a Syk specific artificial peptide substrate capable of detecting physiologically relevant Syk activity in live, intact cells following BCR engagement and oxidative stress using an ELISA-based assay.
MATERIALS AND METHODS
Cell culture and biological reagents
The DG75 and DT40 B cell lines were grown to a density of 0.4 × 106 cells/mL in RPMI-1640 medium containing 7.5% FBS, 1 mM sodium pyruvate, 100 IU/mL penicillin, 100 μg/mL streptomycin and 50 μM 2-metcaptoethanol. Additionally, DT40 cell medium contained 1% chicken serum. Anti-chicken IgM and anti-mouse IgM F(ab′)2 fragments were purchased from Bethyl Laboratories. Anti-human IgM was purchased from Rockland Immunochemicals. Hydrogen peroxide was purchased from Mallinckrodt. Anti-Syk (N-19) was purchased from Santa Cruz Biotechnology. Anti-tubulin and anti-phosphotyrosine (4G10) were purchased from Millipore. Anti-phospho Syk (Y525/526) was purchased from Cell Signaling Technology.
Peptide Synthesis and Purification
The Syk peptide biosensor was synthesized using ‘Fast’ Fmoc solid-phase peptide chemistry with a Prelude Parallel Peptide Synthesizer (Protein Technologies). The synthesized peptides were purified using a C18 reverse-phase column on an Agilent 1200 preparative HPLC system. The peptide was characterized using liquid-chromatography mass spectrometry (LC/MS) on an Accela/LTQ system (Thermo-Finnegan) and matrix-assisted laser desorption/ionization time-of-flight mass spectrometry (MALDI-TOF/TOF) on a Voyager 4800 instrument (Applied Biosystems).
In vitro kinase assay
Recombinant Abl, Src and Lyn enzymes were obtained from a commercial source (Millipore). EGFP-conjugated Syk was isolated from DT40 chicken B cells stably expressing Syk-EGFP. Cells were lysed using a solution containing 1% Nonidet P-40, 50 mM Tris-HCl pH 8.0, 100 mM NaCl, 5 mM EDTA, 1 mM sodium orthovanadate, 2 mM NaF and 1X mammalian protease inhibitor cocktail (Sigma). Syk-EGFP was immunoprecipitated using anti-GFP magnetic nanoparticle beads (MBL, Japan). Lysates were incubated with the beads (40 μl per 1×107 cells) for 1 h at 4°C. The kinase-bound beads were washed and then used in the in vitro kinase assay. Immobilized Syk-EGFP (55 nM per reaction, from 1.2 μg/μl stock, see supporting information Fig. S1), recombinant Lyn, Abl or Src kinase (0.1 U each) was incubated with kinase reaction buffer (500 μM ATP, 5 mM MnCl2, HEPES, pH 7.2) containing the peptide substrate at 25 μM. Aliquots (22 μL) were taken at designated time points and quenched in 0.5 M EDTA, pH 8.5 (22 μL). The quenched sample (1 μL) was diluted into ELISA wash buffer and analyzed as described below. For substrate comparison assays, kinase reaction conditions were as described above except that substrate concentrations were 4 μM, and concentration of enzyme used per reaction was 6 nM. The volume of aliquots diluted in an equal volume of quench buffer was 4 μl (for 8 μl total quenched volume), and the entire quenched amount was diluted into ELISA wash buffer and analyzed as described below. For characterization of SAStide kinetic parameters, substrate concentrations and reaction/quench volumes are given in Table S1.
ELISA-based fluorescence detection
Samples were incubated in a 96-well Neutravidin™ coated plate (15 pmol biotin binding capacity per well, Thermo Scientific) in Tris-buffered saline (TBS) containing 0.1% BSA and 0.05% Tween 20 for 1 h at room temperature on a short-radius plate shaker (600 rpm). Following incubation, wells were washed three times with wash buffer (TBS, 0.1% BSA, 0.05% Tween 20), then incubated with mouse anti-phosphotyrosine monoclonal antibody 4G10 (1:5000 dilution in wash buffer, 100 μL per well) for 1 h at room temperature with shaking. Wells were washed three times with wash buffer and incubated with horseradish peroxidase-conjugated goat anti-mouse immunoglobulin G (IgG) secondary antibody (Abcam) (1:1000 dilution in the wash buffer, 100 μL per well) for 1 h at room temperature with shaking. Wells were then washed three times with wash buffer and twice with sodium phosphate buffer (0.05 M, pH 7.5). For chemifluorescence detection, Amplex Red™ reaction buffer (100 μL total volume/well) consisting of Amplex Red™ reagent (50 μL) (Invitrogen), 20 mM H2O2 (500 μL) and sodium phosphate buffer (4500 μL) and allowed to react for 30 min. Fluorescence of Amplex Red™ was measure using a Synergy4 multiwell plate reader (Biotek) with an excitation wavelength of 532 nm and emission wavelength of 590 nm.
Cell-based peptide biosensor assay
Cells were cultured as described above, harvested and resuspended at a density of 8 × 106 cells/mL (8 mL), then treated with the SAStide biosensor peptide (25 μM) for 15 min prior to stimulation with either anti-IgM antibody (5 μg/mL), H2O2 (3.33 mM) or both. Concentrations of peptide lower than 25 μM resulted in low signal to noise in detection of phosphopeptide using the ELISA-based read-out, and no toxicity was observed in the presence of the peptide at 25 μM (similar to what was observed in our previously published work on Abl kinase16). Aliquots of the cell suspension (1 mL) were harvested, lysed in PhosphoSafe Extraction Reagent (EMD Millipore) containing 167 mM EDTA and freshly prepared protease inhibitor cocktail (Roche) and flash frozen. Half the cell lysate from each sample was used for ELISA-based fluorescence detection and the other for immunoblotting. For dose-response experiments, cells were stimulated with varying concentrations of anti-IgM (2.5–10 μg/mL) or hydrogen peroxide (1–7 mM), harvested at 5 min post-stimulation and processed as described above. For immunoblotting, membranes were blocked in 5% goat serum for 1 h. All primary antibodies were incubated at a dilution of 1:1,000 for 1 h at room temperature and visualized using an HRP-conjugated secondary antibody (Pierce) and ECL reagents (PerkinElmer). Uniformity of the amount of peptide taken up was tested in a representative experiment using Syk-EGFP reconstituted Syk(−/−) DT40 cells, and while there was a very slight (but not statistically significant) trend towards higher peptide amounts over time, no significant difference was seen across conditions (see supporting information, Fig. S3).
Cell-based inhibition assay
Cells (4 × 106 cells/ml) were pre-treated with varying concentrations of piceatannol or dasatinib for 30 min and with the SAStide biosensor peptide (25 μM) for 15 min. Cells were then stimulated with anti-IgM antibody (5 μg/mL) and H2O2 (1 mM) and harvested after 5 min as described above.
Isolation of primary mouse splenic B-cells and primary cell biosensor assay
B cells were enriched from mouse spleens via “panning” as previously described.17 Cells 6 ml, 5 × 106 cells/mL) were treated with vehicle (DMSO), piceatannol (50 μM) or dasatinib (100 nM) for 1 h and with the SAStide biosensor peptide (25 μM) for 15 min prior to stimulation. The cells were stimulated with anti-IgM F(ab′)2 (5 μg/mL). Cells were harvested at 0, 5, 10 and 15 min following stimulation, lysed and analyzed as described above.
RESULTS
Development of a Syk biosensor
Using a Syk substrate consensus motif suggested recently by Geahlen (EXXDEEDYEXPXEPX) and incorporating information on the preferences at the variable sites of that motif gathered from phosphoproteomic studies,11a, 18 we designed a putative Syk peptide substrate, DEEDYEEPDEP, which we named “Syk artificial substrate” or SAStide. We incorporated SAStide along with several functional modules to form a Syk biosensor peptide. These modules include a biotinylated lysine for affinity capture of the substrate, a cell penetrating peptide for delivery of the biosensor into cells and a photocleavable amino acid for release of the substrate from the rest of the biosensor in case mass spectrometry-based analysis is desired for future applications (Figure 1). Phosphorylation of the biosensor by Syk was assessed using an in vitro kinase assay, incubating the SAStide biosensor with immobilized GFP-tagged Syk kinase (immunoprecipitated from DT40 chicken B cells) in the presence of ATP and MnCl2 for varying times after which aliquots of the reaction mixtures were removed and kinase activity quenched by adding an excess of EDTA. Phosphorylated biosensor peptide was detected using a fluorescent ELISA-based assay in which the quenched reaction mixture was incubated in a 96-well Neutravidin™ coated-plate to allow for affinity capture of the biotinylated substrate. Each well had a biotin binding capacity of 15 pmol. The total amount of peptide in the quenched reaction mixture applied to each well was 20 pmol, which ensured that each well was saturated with peptide for analysis. The captured peptide was then incubated with an anti-phosphotyrosine primary antibody (4G10) followed by a horseradish peroxidase-conjugated secondary antibody. Chemifluorescent detection was accomplished by incubating each well with Amplex Red™ reagent and hydrogen peroxide, which gives a fluorescence signal readout that is proportional to the amount of horseradish peroxidase-conjugated antibody in each well, and thus reports the degree of phosphotyrosine present (via the anti-phosphotyrosine antibody) (as described by Wu et al. 19). Relative fluorescence units (RFU) were measured, which provided a proportional representation of the amount of phosphorylated biosensor present. In control experiments to generate a standard curve, phosphorylated SAStide was demonstrated to bind reproducibly to the wells, and exhibited a linear increase in signal up to an amount of phosphopeptide per well of 0.5 pmol (beyond which saturation of the ELISA signal occurred) (Figure S2). This validated that the amount of antiphosphotyrosine antibody-related signal was proportionally related to the degree of peptide phosphorylation. A substantial increase in signal over time was observed, demonstrating that SAStide was phosphorylated by Syk in vitro (Figure 2a). To provide preliminary evidence for the specificity of this substrate for Syk, SAStide was also assayed using Src, Abl and Lyn kinases, none of which produced any significant signal for phosphopeptide (Fig. 2a).
Figure 1.
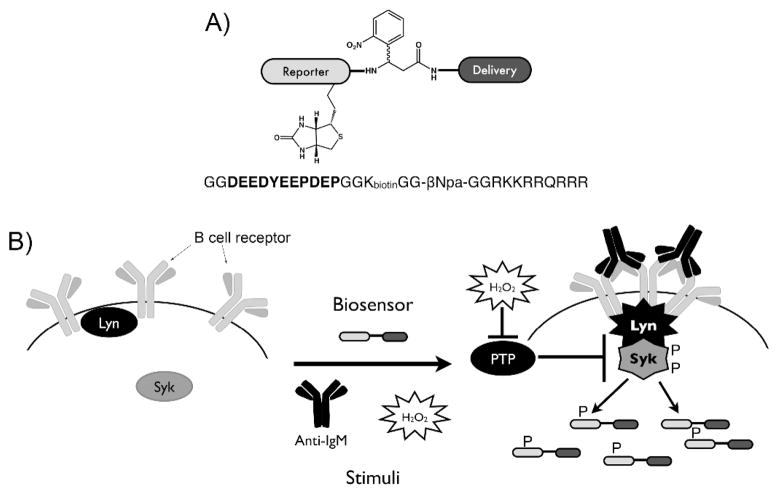
Syk peptide biosensor. The biosensor contains a biotinylated substrate reporter that is phosphorylated by Syk, a photocleavable linker for release of the reporter from the biosensor (for future mass spectrometry applications) and a cell penetrating peptide (TAT peptide) for delivery into live cells. Upon delivery of the biosensor to cells and stimulation of the B cell receptor, the substrate reporter is phosphorylated and can be enriched via the biotin tag and analyzed using an antiphosphotyrosine antibody in an ELISA-style multiwell plate format.
Figure 2.
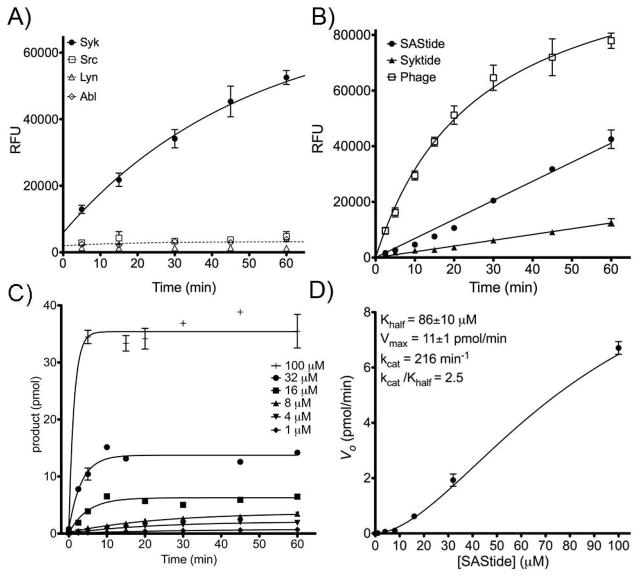
Phosphorylation of the artificial peptide substrate for Syk (SAStide) in vitro. A) The SAStide biosensor (25 μM) was incubated with Syk-EGFP (closed circles), Lyn, Abl or Src in an in vitro kinase assay. Aliquots were removed at designated time points and the amount of phosphorylated substrate was measured using ELISA-based detection (given as relative fluorescence units, RFU). B) SAStide (GGDEEDYEEPDEPGGKbGG) and two known Syk peptide substrates, ‘Syktide’13a (GGEDDEYEEVGGKbGG) and ‘Phage,’13d a peptide derived from a phage display library (GGEDPDYEWPSAGGKbGG), were incubated with immobilized, immunoprecipitated Syk-EGFP kinase (Syk-EGFP) as described in the Materials and Methods. Substrate concentration for each was 4 μM. C) SAStide was assayed with Syk-EGFP at a range of concentrations. D) Initial velocities were calculated from the data in (C) and plotted and fitted to a sigmoidal curve to fit Khalf, Vmax, kcat and kcat/Khalf. For all panels, data points represent the average of three replicate experiments and error bars indicate the standard error of the mean.
We also compared the phosphorylation of SAStide over time with that of two other known substrates of Syk kinase, the natural peptide parent of the previously reported ‘Syktide’ (EDDEYEEV, which in the original work contained an unnatural amino acid in place of the tyrosine that the authors demonstrated conferred specificity for Syk over other kinases e.g. Lyn)13b and a Syk substrate discovered from a phage display library (EDPDYEWPSA).13d Each was synthesized with glycine spacers on the C- and N-terminal sides of the substrate portion with a biotinylated lysine included at the C-terminus for capture and ELISA-based detection of phosphorylation signal (sequences are given in the caption for Fig 2). The kinase assay was performed using immunoprecipitated Syk-EGFP (6 nM) with each substrate at 4 μM. We found that SAStide was phosphorylated more rapidly than the previously reported Syktide sequence, but not as rapidly as the phage display-derived sequence (Fig. 2b). Performing the assay on SAStide at a range of concentrations (Fig. 2c) enabled the characterization of its substrate kinetics with the immunoprecipitated Syk-EGFP enzyme as sigmoidal, indicating some substrate activation through an as-yet unknown mechanism. The kinetic parameters were characterized as Khalf = 86±10 μM (which represents the substrate concentration at half of Vmax in a sigmoidal curve and is analogous to Km) and Vmax = 11±1 pmol/min, from which kcat = 216 min−1 and kcat/Khalf = 2.5 were derived (Fig. 2D). Previously reported for Syktide13b were Km = 3 μM, kcat = 62.2 min−1 and catalytic efficiency kcat/Km = 20.73 (the kinetic parameters for the Phage substrate have not been reported). The SAStide catalytic efficiency is overall comparable to Syktide, with the caveat that the assays were performed with different enzyme preparations and under different substrate (ATP) concentrations. In the direct comparison shown in Fig. 2b (under conditions that emphasize the effects of kcat rather than KM on substrate phosphorylation), SAStide was phosphorylated more efficiently than the parent Syktide. Compared to SAStide, the phage display peptide was more efficiently phosphorylated, but exhibits substrate inhibition at higher concentrations (greater than 20 μM).13d SAStide exhibited no substrate inhibition at concentrations up to 100 μM.
Detection of Dose Dependent Activation and Inhibition of Syk in intact cells
We next examined the ability of the biosensor to detect dose-dependent activation of Syk in the context of BCR activation and oxidative stress in intact, living Burkitt’s lymphoma DG75 B-cells. DG75 cells incubated with the SAStide biosensor were stimulated by cross-linking the BCR using polyclonal antibody specific for IgM11a or by treatment with hydrogen peroxide to inhibit protein tyrosine phosphatases and activate Syk.20 The stimulated cells were harvested and lysed in Phosphosafe extraction buffer with EDTA and protease inhibitors (to prevent further kinase activity, dephosphorylation by phosphatases and proteolytic degradation in the sample), and the lysates were incubated in Neutravidin™-coated wells to capture the biosensor peptide. The chemifluorescent ELISA-based assay described above was used to detect phosphorylation of SAStide. Syk activity was analyzed after 5 min and compared to unstimulated cells as a control. No signal above the reagent background was observed in cells not treated with the peptide. BCR stimulation resulted in increased phosphorylation of the biosensor in a dose-dependent manner (Figure 3A). Induction of oxidative stress in the B cells via hydrogen peroxide treatment also resulted in dose-dependent increases in phosphorylation of the biosensor (Figure 3B). These results show the ability of the peptide biosensor to detect dose-dependent changes in the Syk activity at endogenous levels of expression in live cells.
Figure 3.
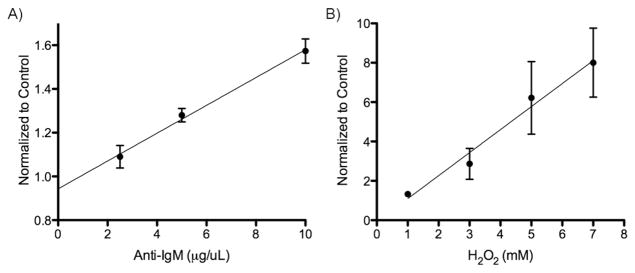
Detection of stimulant dose-dependent intracellular Syk activity. (A) DG75 cells were treated with the SAStide biosensor (25 μM) 15 min prior to stimulation. The cells were stimulated with varying concentrations of anti-IgM (A) or varying concentrations of H2O2 (B). Cells were harvested 5 min following stimulation and the amount of phosphorylated biosensor was measured. Experiments were performed in triplicate and the data are reported as fold change compared to the unstimulated control (which exhibited levels of signal similar to background observed in the in vitro assay shown in Fig. 2, ~4000–8000 RFU). Data points represent the average of three measurements and error bars indicate the standard error of the mean.
We also examined the ability of the biosensor to monitor dose-dependent inhibition of Syk using the Syk-specific natural product inhibitor piceatannol and the Src-family kinase inhibitor, dasatinib (currently being explored as a therapeutic Syk inhibitor21). The inhibitors were assayed in a dilution series from 1 mM – 100 pM (Figure 4A). We found that dasatinib had greater potency than piceatannol for inhibiting Syk phosphorylation of the biosensor. However, as expected from the literature22 and observed from the loss of tubulin and Syk immunoblot signal (Fig. 4B, lanes 17 and 18), high concentrations of piceatannol in the presence of BCR activation and oxidative stress were toxic to DG75 cells whereas dasatinib was not. The apparent IC50 values (the concentration at which SAStide phosphorylation was decreased by 50% compared to the control uninhibited cells) were calculated using the Hill function to be 10.8±9.3 nM and 1.2±1.5 μM for dasatinib and piceatannol, respectively. These results were also consistent with the reduction in the level of Syk tyrosine phosphorylation as detected by Western blot analysis (Figure 4B).
Figure 4.
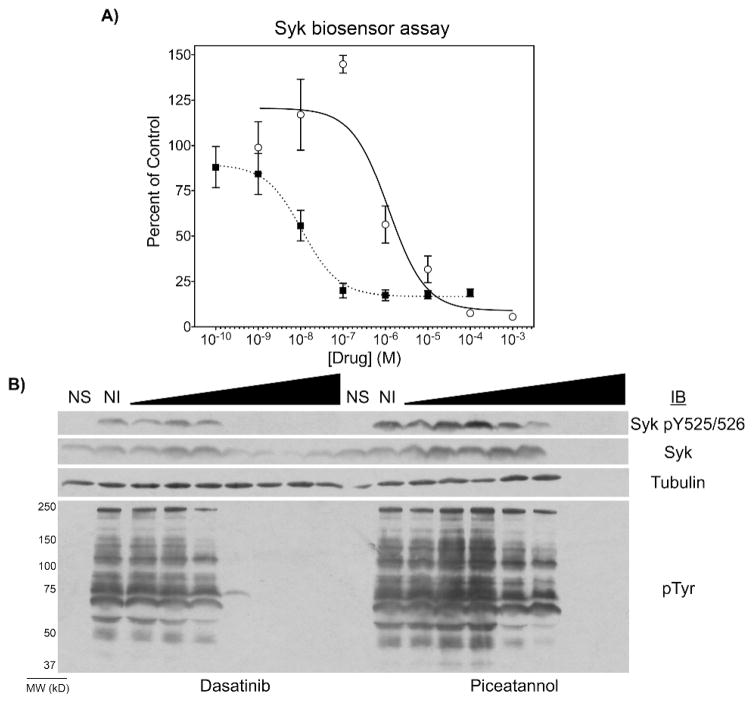
Detection of Syk inhibitor dose-response. Burkitt’s lymphoma DG75 B cells were treated with varying concentrations of dasatinib (closed squares) or piceatannol (open circles) for 30 min and with the SAStide biosensor (25 μM) for 15 min prior to stimulation. The cells were then stimulated with anti-IgM (5 μg/mL) and hydrogen peroxide (1 mM) for 5 min and harvested. (A) The extent of biosensor phosphorylation was analyzed by ELISA. Data indicate averages +/− SEM of experiments performed in triplicate. (B) The level of total Syk, of Syk phosphorylated on Y525 and Y526 (Syk pY525/526) and of tyrosine-phosphorylated proteins (pTyr) were analyzed by Western blotting of lysates of cells not stimulated (NS), stimulated but not treated with inhibitor (NI) or treated with increasing concentrations of the indicated inhibitor. Tubulin was measured as an internal loading control.
Time-dependence of Syk activity following activation
To examine the time dependence of Syk activity following stimulation through the BCR and/or oxidative stress, DG75 cells were treated as above and Syk biosensor phosphorylation was analyzed every few minutes for the first 15 min following stimulation. BCR stimulation gave a rapid increase followed by steadily maintained phosphorylation of the biosensor (Figures 5A and B). As expected, the addition of hydrogen peroxide following BCR stimulation gave a very robust increase in phosphorylation over the time course due to the amplified and extended BCR signaling.11a, 12a, 20 Oxidative stress alone also resulted in increased phosphorylation of the biosensor, peaking at 5 min and subsequently showing a slight decrease.20 The results of this experiment demonstrate that the SAStide biosensor is able to monitor time-dependent increases in Syk activity in live cells following stimulation.
Figure 5.
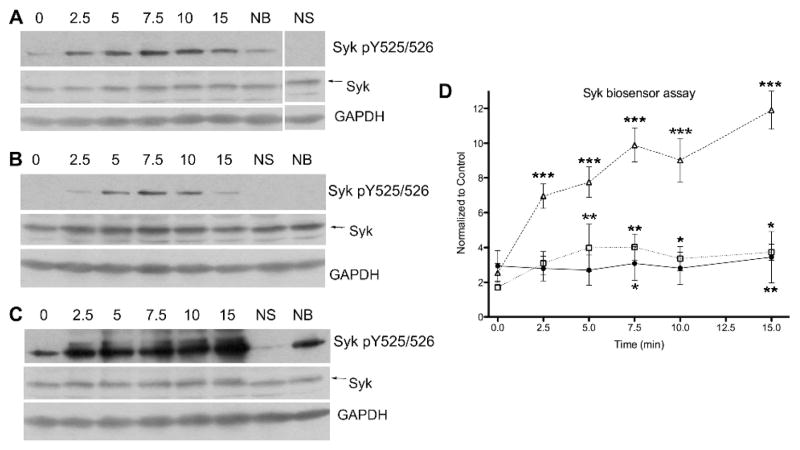
Detection of time-dependent Syk activity following cell stimulation. DG75 B cells were treated with the SAStide biosensor (25 μM) 15 min prior to stimulation. The cells were stimulated with anti-IgM (5 μg/mL) (panel A and closed circles in panel D), H2O2 (3 mM) (panel B and open squares in panel D) or both (5 μg/mL anti-IgM and 3 mM H2O2) (panel C and open triangles in panel D). (A–C), the expression of Syk, Syk phosphorylated on Y525 and Y526 (Syk pY525/526) and GAPDH (loading control) in cell lysates were analyzed by Western blotting. NS - no stimulation; NB - no biosensor (15 min harvest). (D), cells were harvested at varying time points following stimulation and analyzed for biosensor phosphorylation. Experiments were performed in triplicate. Data are reported as normalized change compared to the unstimulated control; error bars are shown either above or below data points for clarity. Data were analyzed using a repeat measure one-way ANOVA test and a Dunnet post-test. Statistical significance is indicated as follows: *P<0.05, **P<0.01 and ***P<0.001.
Determination of the SAStide biosensor specificity in intact cells
While in vitro kinase assays (such as those described in Fig. 2) can provide useful preliminary comparisons of the activity of a kinase for a given substrate, they cannot necessarily be used to unequivocally demonstrate specificity or selectivity. In particular, using classical Michaelis-Menten kinetic parameters such as kcat/Km as “specificity constants” to compare the activity of different enzymes on a single substrate is not relevant since it relies on assumptions that only apply to comparisons of the same enzyme and its interaction with different substrates.23 Therefore, we sought to demonstrate the selective phosphorylation of the SAStide biosensor in the context of the complex intracellular environment. The specificity of the biosensor as a substrate for Syk and not other tyrosine kinases in live cells was explored using DT40 chicken B-cells in which the endogenous gene for Syk has been eliminated by homologous gene targeting.24 Syk-deficient DT40 cells were exposed to the biosensor and stimulated using a polyclonal antibody specific for IgM. As a control, cells were treated with the biosensor but were not stimulated. Phosphorylation of the biosensor was then monitored via quenched aliquots every few minutes for a total of 15 min following stimulation (Fig. 6A). No significant change in phosphorylation of the biosensor was detected over the time course following stimulation when compared to unstimulated control cells. These results indicated that the specificity of the biosensor was maintained in living cells in the context of IgM engagement and BCR-activated signaling.
Figure 6.
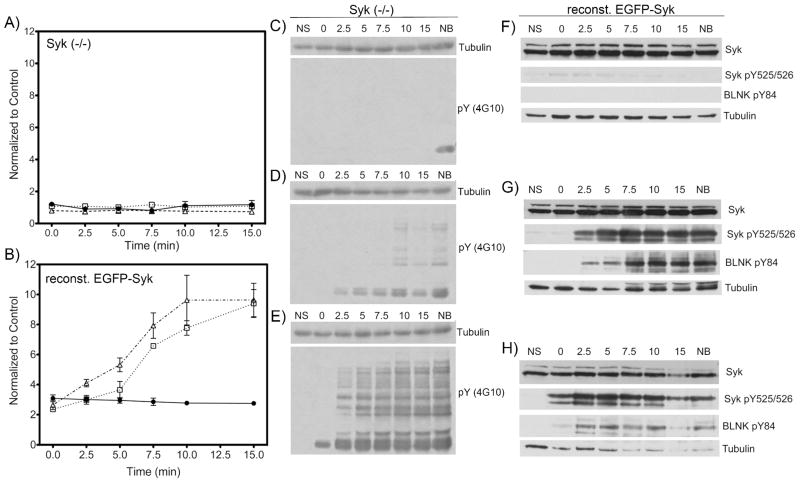
Intracellular specificity of the artificial peptide substrate for Syk. Syk(−/−) DT40 cells or Syk(−/−) DT40 cells reconstituted with stably expressed Syk-EGFP were treated with the SAStide biosensor (25 μM) for 15 min prior to stimulation. The cells were stimulated by treating with anti-IgM (5 μg/mL) (panels C and F, closed circles in graphs A and B), H2O2 (3 mM) (panels D and G, open squares in graphs A and B) or both (5 μg/mL anti-IgM and 3 mM H2O2) (panels E and H, open triangles in graphs A and B), then harvested at varying time points. (C–H): the phosphorylation of proteins in cell lysates was measured by Western blotting using anti-phosphotyrosine (4G10), anti-Syk, antiphospho-Syk(Y525/526), antiphospho-BLNK(Y84) and anti-tubulin antibodies (loading control). NS - no stimulation; NB - no biosensor (15 min harvest). (A, B): chemifluorescence detection of Syk biosensor phosphorylation. The data are reported as normalized change compared to the unstimulated control). Data were analyzed using a repeat measure one-way ANOVA test and a Dunnet post-test; In graph A, no statistically significant difference was seen for any time point or treatment relative to control. Experiments were performed in triplicate.
Since little tyrosine-phosphorylation was observed in anti-IgM-activated, Syk-deficient cells (Figure 6A), BCR-stimulated phosphorylation was amplified by the addition of hydrogen peroxide. As seen with the anti-IgM stimulation alone, no significant change in the phosphorylation of the biosensor was detected over unstimulated cells (Figures 6A). Western blot analysis of tyrosine-phosphorylated proteins demonstrated amplified phosphotyrosine signaling compared to anti-IgM treatment alone, indicating activation of multiple tyrosine kinases and inhibition of tyrosine phosphatases (Figure 6E). Similarly, no significant change in the phosphorylation of the biosensor was detected in cells treated only with hydrogen peroxide (Figures 6D). This showed that even in the presence of amplified and extended BCR-related and other H2O2-related signaling the SAStide biosensor was not appreciably phosphorylated by other highly activated tyrosine kinases in Syk-deficient cells. This experiment serves as a highly relevant specificity control in the intracellular context, given that tyrosine kinase activity was clearly upregulated, yet none of these activated kinases phosphorylated the biosensor peptide.
To further support specificity, we performed the same set of experiments in Syk (−/−) DT40 cells that had been reconstituted with Syk-EGFP. In the presence of BCR stimulation by IgM, signal indicating phosphorylation of the biosensor peptide was increased approximately 2-fold over control (unstimulated Syk-EGFP-expressing cells) and decreased slightly over time (Fig. 6B). When treated with H2O2 with or without concurrent BCR stimulation, biosensor phosphorylation signal increased more dramatically (to approximately 12-fold over control) (Fig. 6B). Phosphorylation of the biosensor was consistent with that observed for the Syk autophosphorylation site and the known Syk substrate BLNK (Fig. 6F–H).
Detection of Syk Activity and Inhibition in Primary Mouse Splenic B-cells
To examine the ability of the biosensor to monitor Syk activity and response to inhibitor treatment in primary cells expressing endogenous levels of Syk, we isolated mouse primary splenic B cells and treated these with the biosensor peptide in the presence of the Syk inhibitors piceatannol or dasatinib. Primary B cells were treated with 10 nM dasatinib, 1 μM piceatannol or vehicle (DMSO) for one h prior to stimulation. The SAStide biosensor peptide was added 15 min prior to stimulation, and the cells were then treated with anti-IgM F(ab′)2 (5 μg/mL) and harvested at 0, 5, 10 and 15 min following stimulation. As seen with the DG75 cells, stimulation of Syk activity following BCR engagement resulted in a rapid increase in phosphorylation of the SAStide biosensor (within approximately two minutes—the time required to handle and process an aliquot of cells collected immediately after stimulation) followed by a maintenance of phosphorylation over time as compared to control unstimulated cells, which showed only background levels of phosphorylation-related signal (Fig. 7). In the presence of each inhibitor the level of phosphorylation of the biosensor was decreased, giving a signal that was close to background levels. These results showed that the biosensor peptide was capable of detecting the activation and inhibition of endogenous Syk kinase expressed at normal levels in primary cells that exhibit physiologically relevant B cell receptor signaling, and suggest that elevation of Syk activity is very rapid in response to B cell receptor engagement in these primary cells.
Figure 7.
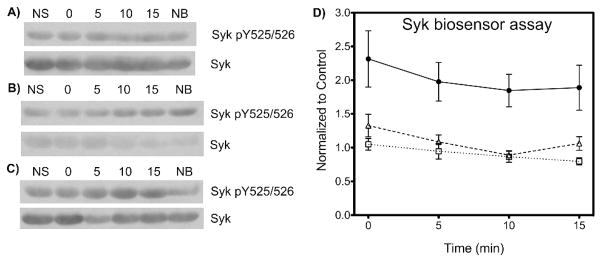
Detection of Syk activity and inhibition in mouse primary splenic B-cells. Mouse primary splenic B-cells were treated without (panel A and closed circles in panel D) or with the tyrosine kinase inhibitors dasatinib (100 nM) (B), (D △) or piceatannol (50 μM) (C), (D □) for 30 min prior to stimulation, then treated with the SAStide biosensor (25 μM, 15 min) and stimulated with anti-IgM F(ab′)2 (5 μg/μL). Cells were harvested at various time points following stimulation. (A–C) the expression of Syk and of Syk phosphorylated on Y525 and Y526 (Syk pY525/526) were determined by Western blotting. NS - no stimulation; NB - no biosensor (15 min harvest). (D) The data are reported as normalized change in chemifluorescence signal compared to the unstimulated control. N = 6; error bars indicate SEM.
DISCUSSION
These studies present the development of a new versatile tool for direct and specific monitoring of intracellular Syk kinase activity in physiological contexts. We showed that we could develop a Syk-specific biosensor peptide by combining a peptide based on a Syk substrate consensus sequence11a, 18a with other modular units including a biotinylated lysine for affinity capture of the substrate and a cell penetrating peptide for delivery of the biosensor into cells. Similar to the Abl kinase biosensor we previously reported,16 this Syk kinase peptide biosensor (SAStide) did not cause toxicity at the concentration used in these studies, and was able to detect dose-dependent and time-dependent activation and inhibition of endogenous Syk using physiologically relevant stimuli in cultured cell lines as well as primary splenic mouse B cells. These results demonstrate the potential for this strategy to be used in a multiwell plate ELISA assay to analyze Syk activity in contexts that could include study of signaling processes in a basic research setting or even potentially monitoring therapeutic response in translational applications.
Crucially, specificity of the SAStide biosensor for detecting Syk activation was comprehensively demonstrated in a complex, biologically-relevant system using Syk-deficient DT40 chicken B cells. Syk plays a key role in B cell signaling, but is partly dependent upon activation of Lyn through antigen binding to the BCR. Stimulation of Syk-deficient cells by cross-linking the heavy chain of the BCR in the presence and absence of H2O2 allowed for the specificity of the biosensor to be assessed in the context of Lyn kinase activation. Phosphorylation of the biosensor was not increased above background levels and did not change over time following BCR engagement in the absence of Syk, even when overall tyrosine kinase signaling was amplified as a result of H2O2 exposure. Syk also plays a major role in oxidative stress signaling, and its activation during oxidative stress is the result of both its own activity (via autophosphorylation) as well as other protein tyrosine kinases.20a, 25 Other kinases also become activated during oxidative stress (as observed via antiphosphotyrosine blotting), yet there was no increase in phosphorylation of the biosensor in the absence of Syk under these conditions. Reconstitution of the Syk deficient cells with Syk-EGFP resulted in phosphorylation of the biosensor peptide under B cell receptor-activating conditions and in the presence of oxidative stress. This suggests that even in a complex cellular environment, our biosensor is selectively phosphorylated by Syk and not by Lyn or other activated tyrosine kinases in these cells. Together these results demonstrate that our biosensor is specific for the detection of Syk activity in B cells and B cell model systems.
Another advantage of this strategy is the ability to monitor kinase activation and inhibition by compounds such as piceatannol and dasatinib in an intact cell. Isolation of a kinase from the cellular environment can alter its function by removing regulatory proteins, eliminating alternatively spliced variants, altering post-translational modifications and/or disrupting subcellular compartmentalization. Additionally, isolation of the kinase precludes evaluation of the contribution of off-target effects of the drug, which could potentially affect efficacy (positively or negatively) via inhibition of upstream signaling in addition to the direct inhibition of the target. While kinase activation in intact cell contexts has been studied by detecting the phosphorylation of known endogenous substrate sites, unambiguous detection of these sites requires high-quality phospho-specific antibodies, and furthermore, not every endogenous site can serve as a reliable generic ‘marker’ for the kinase activation state. For Syk, monitoring phosphorylation of its tyrosines through surrogate sites (Y348, Y352, Y525/526) has been the traditional strategy for determining its activation11a, 21—however, this does not necessarily give an accurate report of Syk activity, since some of these sites are phosphorylated by upstream kinases and some by Syk autophosphorylation, and moreover, phosphorylation of some of these sites modifies the function of the kinase while others do not. While Y525/526 is in the activation loop of Syk and is a major autophosphorylation site in B cells26 and canonically phosphorylation of these sites would suggest enhancement of kinase activity, the role of this phosphorylation in the functional activation of Syk remains convoluted. Previous work using a Sox-based fluorogenic, generic tyrosine kinase substrate from Invitrogen has demonstrated that substrate phosphorylation kinetics by activation loop-phosphorylated Syk kinase domain (i.e. the phospho-Y525/526 form) are similar to those for the unphosphorylated and double phenylalanine mutant (F525/F526) forms.14 This suggests that these residues are not accurate markers of intrinsic kinase activation, and that a selective tool that can monitor Syk’s activation state in complex cellular environments independently of these existing markers would be useful for delineating the effects of specific phosphorylation sites and kinase inhibitors on different components of the B cell receptor signaling cascade.
Using the SAStide biosensor, we also detected inhibition of endogenous intracellular Syk activity in a dose-dependent manner by the Src-family kinase inhibitors, dasatinib, and piceatannol. We found that the apparent IC50 values were 10 nM and 1 μM for dasatinib and piceatannol, respectively. These values are lower than those reported in the literature,21, 27 however the literature values were determined using assay formats that were very different than the format employed here, and so cannot necessarily be directly compared. When examining Syk activation in the mouse primary B cells in the presence of these inhibitors, we observed levels of biosensor phosphorylation consistent with those observed in EGFP-reconstituted DT40-Syk(−/−) cells—however, according to Western blot analysis, Y525/526 remained phosphorylated under these conditions. Since these cells were isolated from the spleen, this could be related to the role of kinases acting upstream of Syk in signaling pathways.28 Along with the previous report that Y525/526 to Phe mutations did not affect the kinase activity, these data also support the idea that monitoring an endogenous phosphorylation site as a surrogate for Syk activity does not necessarily correctly report the activity of the kinase or response to the inhibitor treatment—whereas phosphorylation of this SAStide biosensor may be used as a marker for intrinsic activation of the kinase.
Aside from its potential utility in basic research on the function of Syk kinase, the straightforward workflow and compatibility of this biosensor substrate with the multiwell ELISA-style readout might be useful in a translational setting to determine Syk kinase pharmacodynamics in patient B cell populations. Despite the observations from the basic science research that Syk dysfunction plays a role in disease, the outcomes of clinical studies and trials for Syk inhibitors indicate that the benefits of blocking Syk activity in patients are less clear. A recent report of results from an ongoing clinical trial in non-Hodgkins lymphomas (#NCT00446095) of the Syk inhibitor fostamatinib (R788, a prodrug form of R406, Rigel Pharmaceuticals) described a range of effects depending on lymphoma subtype, which included DLBCL, follicular lymphoma, mantle cell lymphoma, MALT lymphoma, marginal zone lymphoma, CLL and SLL.29 The cohort was very small, particularly when dissected by lymphoma subtype, and response rates were low overall, with slightly better response in chronic lymphocytic leukemia than the other types. Notably, in a recent study the R406 form of this drug was found to be one of the least selective kinase inhibitors evaluated, in terms of binding affinity for kinases.30 This highlights the need for pharmacodynamically-relevant assays during the evaluation and development of so-called “specific” kinase inhibitors.
On the immune side, another Syk inhibitor (R112, Rigel Pharmaceuticals), was shown to be effective for relieving allergic rhinitis symptoms when delivered intranasally, suggesting that there could be a therapeutic benefit to targeting Syk (which is upstream of histamine release) for this purpose, and thus may provide an alternative to antihistamine drugs.31 For rheumatoid arthritis, an earlier trial found that Fostamatinib provided a significant benefit (over placebo) for rheumatoid arthritis patients receiving methotrexate;32 however, a more recent follow-up study by the same group found that this inhibitor was not significantly beneficial in another subset of patients who had previously failed biologic therapy (e.g. rituximab or abatacept).33 As the authors noted, their “study was fraught with seemingly contradictory data.” Many other factors in these patients’ diseases (such as status of other inflammatory markers and ability to assess response metrics in the presence of confounding factors) may have contributed to the overall assessment of responses. Another intriguing possibility is that initial response to biologics requires Syk involvement, and therefore this patient subset is inherently unlikely to respond to an alternative Syk-targeting strategy. This is also consistent with evidence that lower Syk expression in acute myelogenous leukemia is related to a lack of response to another therapeutic antibody conjugated to a toxin (gemtuzumab ozogamicin).34 Either way, this collection of paradoxical and weak responses indicates that monitoring Syk kinase activity and function in the context of individual patients could aid the interpretation of outcomes for trials of Syk-targeting agents, and maybe someday help guide patient stratification for likelihood of benefit. This will be increasingly important as new, more functionally selective Syk inhibitors (e.g. the recently reported compound P505-153a) are brought into the market and through clinical trials.
Supplementary Material
Footnotes
This work was supported by the National Institutes of Health, National Cancer Institute R25CA128770 (D. Teegarden) Cancer Prevention Internship Program (A.M.L) administered by the Oncological Sciences Center and the Discovery Learning Research Center at Purdue University, through a K99/R00 Pathway to Independence award to L.L.P (CA127161), and NIH grant CA037372 and AI098132 (R.L.G.).
Supporting Information Available. Supplemental materials may be accessed free of charge online at http://pubs.acs.org. Characterization of immunoprecipitated Syk-EGFP preparations, standard curves for the in vitro kinase assay and demonstration of uniformity of total peptide taken up by cells are provided.
REFEENCES
- 1.(a) Kobayashi T, Nakamura S, Taniguchi T, Yamamura H. Purification and characterization of a cytosolic protein-tyrosine kinase from porcine spleen. European journal of biochemistry/FEBS. 1990;188(3):535–540. doi: 10.1111/j.1432-1033.1990.tb15433.x. [DOI] [PubMed] [Google Scholar]; (b) Zioncheck TF, Harrison ML, Geahlen RL. Purification and characterization of a protein-tyrosine kinase from bovine thymus. The Journal of biological chemistry. 1986;261(33):15637–15643. [PubMed] [Google Scholar]
- 2.Turner M, Mee PJ, Costello PS, Williams O, Price AA, Duddy LP, Furlong MT, Geahlen RL, Tybulewicz VL. Perinatal lethality and blocked B-cell development in mice lacking the tyrosine kinase Syk. Nature. 1995;378(6554):298–302. doi: 10.1038/378298a0. [DOI] [PubMed] [Google Scholar]
- 3.(a) Coffey G, DeGuzman F, Inagaki M, Pak Y, Delaney SM, Ives D, Betz A, Jia ZJ, Pandey A, Baker D, Hollenbach SJ, Phillips DR, Sinha U. Specific inhibition of spleen tyrosine kinase suppresses leukocyte immune function and inflammation in animal models of rheumatoid arthritis. The Journal of pharmacology and experimental therapeutics. 2012;340(2):350–359. doi: 10.1124/jpet.111.188441. [DOI] [PubMed] [Google Scholar]; (b) Hirabayashi A, Mukaiyama H, Kobayashi H, Shiohara H, Nakayama S, Ozawa M, Miyazawa K, Misawa K, Ohnota H, Isaji M. A novel Syk family kinase inhibitor: design, synthesis, and structure-activity relationship of 1,2,4-triazolo[4,3-c]pyrimidine and 1,2,4-triazolo[1,5-c]pyrimidine derivatives. Bioorganic & medicinal chemistry. 2008;16(15):7347–7357. doi: 10.1016/j.bmc.2008.06.017. [DOI] [PubMed] [Google Scholar]; (c) Lai JY, Cox PJ, Patel R, Sadiq S, Aldous DJ, Thurairatnam S, Smith K, Wheeler D, Jagpal S, Parveen S, Fenton G, Harrison TK, McCarthy C, Bamborough P. Potent small molecule inhibitors of spleen tyrosine kinase (Syk) Bioorganic & medicinal chemistry letters. 2003;13(18):3111–3114. doi: 10.1016/s0960-894x(03)00658-9. [DOI] [PubMed] [Google Scholar]; (d) Yamamoto N, Takeshita K, Shichijo M, Kokubo T, Sato M, Nakashima K, Ishimori M, Nagai H, Li YF, Yura T, Bacon KB. The orally available spleen tyrosine kinase inhibitor 2-[7-(3,4-dimethoxyphenyl)-imidazo[1,2-c]pyrimidin-5-ylamino]nicotinamide dihydrochloride (BAY 61-3606) blocks antigen-induced airway inflammation in rodents. The Journal of pharmacology and experimental therapeutics. 2003;306(3):1174–1181. doi: 10.1124/jpet.103.052316. [DOI] [PubMed] [Google Scholar]
- 4.Hahn CK, Berchuck JE, Ross KN, Kakoza RM, Clauser K, Schinzel AC, Ross L, Galinsky I, Davis TN, Silver SJ, Root DE, Stone RM, DeAngelo DJ, Carroll M, Hahn WC, Carr SA, Golub TR, Kung AL, Stegmaier K. Proteomic and genetic approaches identify Syk as an AML target. Cancer cell. 2009;16(4):281–294. doi: 10.1016/j.ccr.2009.08.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Semichon M, Merle-Beral H, Lang V, Bismuth G. Normal Syk protein level but abnormal tyrosine phosphorylation in B-CLL cells. Leukemia : official journal of the Leukemia Society of America, Leukemia Research Fund, UK. 1997;11(11):1921–1928. doi: 10.1038/sj.leu.2400832. [DOI] [PubMed] [Google Scholar]
- 6.Baudot AD, Jeandel PY, Mouska X, Maurer U, Tartare-Deckert S, Raynaud SD, Cassuto JP, Ticchioni M, Deckert M. The tyrosine kinase Syk regulates the survival of chronic lymphocytic leukemia B cells through PKCdelta and proteasome-dependent regulation of Mcl-1 expression. Oncogene. 2009;28(37):3261–3273. doi: 10.1038/onc.2009.179. [DOI] [PubMed] [Google Scholar]
- 7.Feldman AL, Sun DX, Law ME, Novak AJ, Attygalle AD, Thorland EC, Fink SR, Vrana JA, Caron BL, Morice WG, Remstein ED, Grogg KL, Kurtin PJ, Macon WR, Dogan A. Overexpression of Syk tyrosine kinase in peripheral T-cell lymphomas. Leukemia : official journal of the Leukemia Society of America, Leukemia Research Fund, UK. 2008;22(6):1139–1143. doi: 10.1038/leu.2008.77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Wilcox RA, Sun DX, Novak A, Dogan A, Ansell SM, Feldman AL. Inhibition of Syk protein tyrosine kinase induces apoptosis and blocks proliferation in T-cell non-Hodgkin’s lymphoma cell lines. Leukemia : official journal of the Leukemia Society of America, Leukemia Research Fund, UK. 2010;24(1):229–232. doi: 10.1038/leu.2009.198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Coopman PJ, Do MT, Barth M, Bowden ET, Hayes AJ, Basyuk E, Blancato JK, Vezza PR, McLeskey SW, Mangeat PH, Mueller SC. The Syk tyrosine kinase suppresses malignant growth of human breast cancer cells. Nature. 2000;406(6797):742–747. doi: 10.1038/35021086. [DOI] [PubMed] [Google Scholar]
- 10.Sung YM, Xu X, Sun J, Mueller D, Sentissi K, Johnson P, Urbach E, Seillier-Moiseiwitsch F, Johnson MD, Mueller SC. Tumor suppressor function of Syk in human MCF10A in vitro and normal mouse mammary epithelium in vivo. PloS one. 2009;4(10):e7445. doi: 10.1371/journal.pone.0007445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.(a) Geahlen RL. Syk and pTyr’d: Signaling through the B cell antigen receptor. Biochimica et biophysica acta. 2009;1793(7):1115–1127. doi: 10.1016/j.bbamcr.2009.03.004. [DOI] [PMC free article] [PubMed] [Google Scholar]; (b) Lupher ML, Jr, Rao N, Lill NL, Andoniou CE, Miyake S, Clark EA, Druker B, Band H. Cbl-mediated negative regulation of the Syk tyrosine kinase. A critical role for Cbl phosphotyrosine-binding domain binding to Syk phosphotyrosine 323. The Journal of biological chemistry. 1998;273(52):35273–35281. doi: 10.1074/jbc.273.52.35273. [DOI] [PubMed] [Google Scholar]
- 12.(a) Irish JM, Czerwinski DK, Nolan GP, Levy R. Altered B-cell receptor signaling kinetics distinguish human follicular lymphoma B cells from tumor-infiltrating nonmalignant B cells. Blood. 2006;108(9):3135–3142. doi: 10.1182/blood-2006-02-003921. [DOI] [PMC free article] [PubMed] [Google Scholar]; (b) Irish JM, Czerwinski DK, Nolan GP, Levy R. Kinetics of B cell receptor signaling in human B cell subsets mapped by phosphospecific flow cytometry. J Immunol. 2006;177(3):1581–1589. doi: 10.4049/jimmunol.177.3.1581. [DOI] [PubMed] [Google Scholar]
- 13.(a) Brunati AM, Donella-Deana A, Ruzzene M, Marin O, Pinna LA. Site specificity of p72syk protein tyrosine kinase: efficient phosphorylation of motifs recognized by Src homology 2 domains of the Src family. FEBS letters. 1995;367(2):149–152. doi: 10.1016/0014-5793(95)00555-n. [DOI] [PubMed] [Google Scholar]; (b) Donella-Deana A, Ruzza P, Cesaro L, Brunati AM, Calderan A, Borin G, Pinna LA. Specific monitoring of Syk protein kinase activity by peptide substrates including constrained analogs of tyrosine. FEBS letters. 2002;523(1–3):48–52. doi: 10.1016/s0014-5793(02)02932-0. [DOI] [PubMed] [Google Scholar]; (c) Li M, Luraghi P, Amour A, Qian XD, Carter PS, Clark CJ, Deakin A, Denyer J, Hobbs CI, Surby M, Patel VK, Schaefer EM. Kinetic assay for characterization of spleen tyrosine kinase activity and inhibition with recombinant kinase and crude cell lysates. Anal Biochem. 2009;384(1):56–67. doi: 10.1016/j.ab.2008.07.040. [DOI] [PubMed] [Google Scholar]; (d) Schmitz R, Baumann G, Gram H. Catalytic specificity of phosphotyrosine kinases Blk, Lyn, c-Src and Syk as assessed by phage display. Journal of molecular biology. 1996;260(5):664–677. doi: 10.1006/jmbi.1996.0429. [DOI] [PubMed] [Google Scholar]
- 14.Papp E, Tse JK, Ho H, Wang S, Shaw D, Lee S, Barnett J, Swinney DC, Bradshaw JM. Steady state kinetics of spleen tyrosine kinase investigated by a real time fluorescence assay. Biochemistry. 2007;46(51):15103–15114. doi: 10.1021/bi701596u. [DOI] [PubMed] [Google Scholar]
- 15.(a) Li H, Sims CE, Kaluzova M, Stanbridge EJ, Allbritton NL. A quantitative single-cell assay for protein kinase B reveals important insights into the biochemical behavior of an intracellular substrate peptide. Biochemistry. 2004;43(6):1599–1608. doi: 10.1021/bi035597k. [DOI] [PubMed] [Google Scholar]; (b) Soughayer JS, Wang Y, Li H, Cheung SH, Rossi FM, Stanbridge EJ, Sims CE, Allbritton NL. Characterization of TAT-mediated transport of detachable kinase substrates. Biochemistry. 2004;43(26):8528–8540. doi: 10.1021/bi036296d. [DOI] [PubMed] [Google Scholar]
- 16.(a) Placzek EA, Plebanek MP, Lipchik AM, Kidd SR, Parker LL. A peptide biosensor for detecting intracellular Abl kinase activity using matrix-assisted laser desorption/ionization time-of-flight mass spectrometry. Anal Biochem. 2010;397(1):73–78. doi: 10.1016/j.ab.2009.09.048. [DOI] [PMC free article] [PubMed] [Google Scholar]; (b) Tang J, Wang JY, Parker LL. Detection of early abl kinase activation after ionizing radiation by using a Peptide biosensor. Chembiochem : a European journal of chemical biology. 2012;13(5):665–673. doi: 10.1002/cbic.201100763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Severson CD, Burg DL, Lafrenz DE, Feldbush TL. An alternative method of panning for rat B lymphocytes. Immunol Lett. 1987;15(4):291–295. doi: 10.1016/0165-2478(87)90130-1. [DOI] [PubMed] [Google Scholar]
- 18.(a) Iliuk AB, Martin VA, Alicie BM, Geahlen RL, Tao WA. In-depth analyses of kinase-dependent tyrosine phosphoproteomes based on metal ion-functionalized soluble nanopolymers. Molecular & cellular proteomics : MCP. 2010;9(10):2162–2172. doi: 10.1074/mcp.M110.000091. [DOI] [PMC free article] [PubMed] [Google Scholar]; (a) Xue L, Wang WH, Iliuk A, Hu L, Galan JA, Yu S, Hans M, Geahlen RL, Tao WA. Sensitive kinase assay linked with phosphoproteomics for identifying direct kinase substrates; Proceedings of the National Academy of Sciences of the United States of America; 2012. pp. 5615–5620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Wu D, Mand MR, Veach DR, Parker LL, Clarkson B, Kron SJ. A solid-phase Bcr-Abl kinase assay in 96-well hydrogel plates. Anal Biochem. 2008;375(1):18–26. doi: 10.1016/j.ab.2007.12.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.(a) Qin S, Kurosaki T, Yamamura H. Differential regulation of oxidative and osmotic stress induced Syk activation by both autophosphorylation and SH2 domains. Biochemistry. 1998;37(16):5481–5486. doi: 10.1021/bi9729460. [DOI] [PubMed] [Google Scholar]; (b) Qin S, Minami Y, Hibi M, Kurosaki T, Yamamura H. Syk-dependent and -independent signaling cascades in B cells elicited by osmotic and oxidative stress. The Journal of biological chemistry. 1997;272(4):2098–2103. doi: 10.1074/jbc.272.4.2098. [DOI] [PubMed] [Google Scholar]
- 21.McCaig AM, Cosimo E, Leach MT, Michie AM. Dasatinib inhibits B cell receptor signalling in chronic lymphocytic leukaemia but novel combination approaches are required to overcome additional pro-survival microenvironmental signals. British journal of haematology. 2011:199–211. doi: 10.1111/j.1365-2141.2010.08507.x. [DOI] [PubMed] [Google Scholar]
- 22.Piotrowska H, Kucinska M, Murias M. Biological activity of piceatannol: leaving the shadow of resveratrol. Mutation research. 2012;750(1):60–82. doi: 10.1016/j.mrrev.2011.11.001. [DOI] [PubMed] [Google Scholar]
- 23.Eisenthal R, Danson MJ, Hough DW. Catalytic efficiency and kcat/KM: a useful comparator? Trends in biotechnology. 2007;25(6):247–249. doi: 10.1016/j.tibtech.2007.03.010. [DOI] [PubMed] [Google Scholar]
- 24.Takata M, Sabe H, Hata A, Inazu T, Homma Y, Nukada T, Yamamura H, Kurosaki T. Tyrosine kinases Lyn and Syk regulate B cell receptor-coupled Ca2+ mobilization through distinct pathways. The EMBO journal. 1994;13(6):1341–1349. doi: 10.1002/j.1460-2075.1994.tb06387.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Schieven GL, Kirihara JM, Burg DL, Geahlen RL, Ledbetter JA. p72syk tyrosine kinase is activated by oxidizing conditions that induce lymphocyte tyrosine phosphorylation and Ca2+ signals. The Journal of biological chemistry. 1993;268(22):16688–16692. [PubMed] [Google Scholar]
- 26.Keshvara LM, Isaacson CC, Yankee TM, Sarac R, Harrison ML, Geahlen RL. Syk- and Lyn-dependent phosphorylation of Syk on multiple tyrosines following B cell activation includes a site that negatively regulates signaling. J Immunol. 1998;161(10):5276–5283. [PubMed] [Google Scholar]
- 27.Geahlen RL, McLaughlin JL. Piceatannol (3,4,3′,5′-tetrahydroxy-trans-stilbene) is a naturally occurring protein-tyrosine kinase inhibitor. Biochemical and biophysical research communications. 1989;165(1):241–245. doi: 10.1016/0006-291x(89)91060-7. [DOI] [PubMed] [Google Scholar]
- 28.Miller LA, Hong JJ, Kinch MS, Harrison ML, Geahlen RL. The engagement of beta1 integrins on promonocytic cells promotes phosphorylation of Syk and formation of a protein complex containing Lyn and beta1 integrin. European journal of immunology. 1999;29(5):1426–1434. doi: 10.1002/(SICI)1521-4141(199905)29:05<1426::AID-IMMU1426>3.0.CO;2-J. [DOI] [PubMed] [Google Scholar]
- 29.Friedberg JW, Sharman J, Sweetenham J, Johnston PB, Vose JM, Lacasce A, Schaefer-Cutillo J, De Vos S, Sinha R, Leonard JP, Cripe LD, Gregory SA, Sterba MP, Lowe AM, Levy R, Shipp MA. Inhibition of Syk with fostamatinib disodium has significant clinical activity in non-Hodgkin lymphoma and chronic lymphocytic leukemia. Blood. 2010;115(13):2578–2585. doi: 10.1182/blood-2009-08-236471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Davis MI, Hunt JP, Herrgard S, Ciceri P, Wodicka LM, Pallares G, Hocker M, Treiber DK, Zarrinkar PP. Comprehensive analysis of kinase inhibitor selectivity. Nature biotechnology. 2011;29(11):1046–1051. doi: 10.1038/nbt.1990. [DOI] [PubMed] [Google Scholar]
- 31.Meltzer EO, Berkowitz RB, Grossbard EB. An intranasal Syk-kinase inhibitor (R112) improves the symptoms of seasonal allergic rhinitis in a park environment. The Journal of allergy and clinical immunology. 2005;115(4):791–796. doi: 10.1016/j.jaci.2005.01.040. [DOI] [PubMed] [Google Scholar]
- 32.Weinblatt ME, Kavanaugh A, Burgos-Vargas R, Dikranian AH, Medrano-Ramirez G, Morales-Torres JL, Murphy FT, Musser TK, Straniero N, Vicente-Gonzales AV, Grossbard E. Treatment of rheumatoid arthritis with a Syk kinase inhibitor: a twelve-week, randomized, placebo-controlled trial. Arthritis and rheumatism. 2008;58(11):3309–3318. doi: 10.1002/art.23992. [DOI] [PubMed] [Google Scholar]
- 33.Genovese MC, Kavanaugh A, Weinblatt ME, Peterfy C, DiCarlo J, White ML, O’Brien M, Grossbard EB, Magilavy DB. An oral Syk kinase inhibitor in the treatment of rheumatoid arthritis: a three-month randomized, placebo-controlled, phase II study in patients with active rheumatoid arthritis that did not respond to biologic agents. Arthritis and rheumatism. 2011;63(2):337–345. doi: 10.1002/art.30114. [DOI] [PubMed] [Google Scholar]
- 34.Balaian L, Ball ED. Cytotoxic activity of gemtuzumab ozogamicin (Mylotarg) in acute myeloid leukemia correlates with the expression of protein kinase Syk. Leukemia : official journal of the Leukemia Society of America, Leukemia Research Fund, UK. 2006;20(12):2093–2101. doi: 10.1038/sj.leu.2404437. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


