Figure 9. Two roles for YopK in controlling the injectisome.
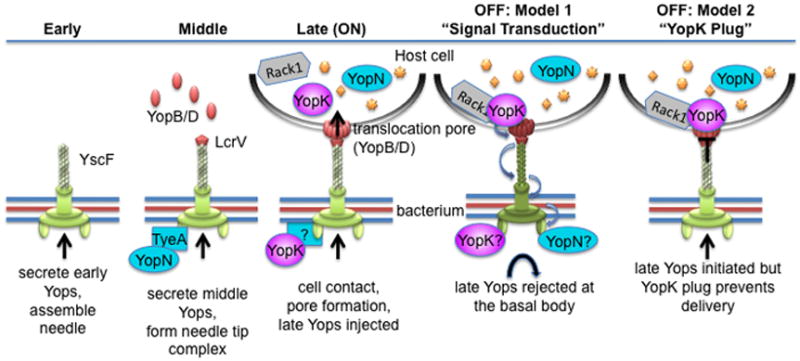
A model is shown to depict basic stages of injectisome assembly, activation, and deactivation. In the “early” stage, needle subunit (YscF) and other early substrates needed for needle assembly are secreted. Reaching optimal needle length triggers a substrate specificity switch and transition to the “middle” stage. This allows secretion of the translocators and assembly of the needle tip complex. Upon contact with a host cell, the translocators form a pore in the host membrane that completes the channel from the bacterium into the host. Channel completion coincides with transition to the “late” stage, in which the injectisome is “ON” and now injects effector Yops (late Yops) directly into host cells. During this time YopK accumulates in host cells and interacts with Rack1 and the translocation pore, and the end result is the down regulation of Yop injection (OFF state). In the first model, YopK would interact with the translocation pore, triggering a cascade of conformational changes throughout the injectisome, which ultimately prevent initiation of further Yop injection. In the second model, YopK would bind to the translocation pore and act as a cap that closes the channel to prevent further Yop injection.
