Fig. 12.
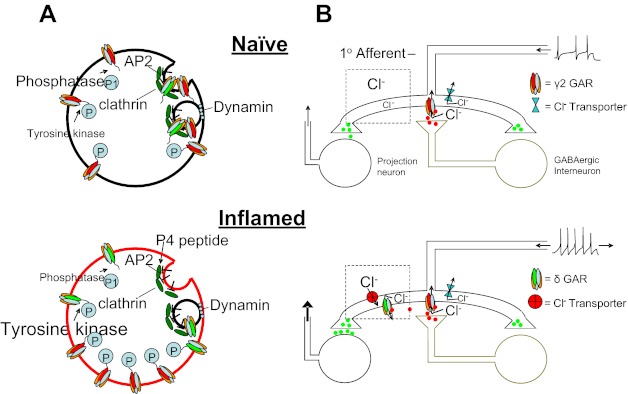
Model of inflammation-induced changes in GABAA receptor (GAR) signaling. A: at a cellular level, in the absence of inflammation (top), the relative level of tyrosine kinase activity is low. Consequently, turnover of GABAA receptors is high, with many receptors internalized in clathrin-coated pits via a dynamin-dependent process. In the presence of inflammation (bottom), there is an increase in the relative level of tyrosine kinase activity and consequently an increase in the phosphorylation of GABAA receptors. As this phosphorylation of receptors can mask an AP2 binding site, internalization of GABAA receptors is decreased and current density is increased. Application of genistein reduces tyrosine kinase activity, resulting in a decrease in phosphorylation of GABAA receptors, an increase in receptor internalization, and a decrease in current. The P4 peptide is designed to block the binding of dynamin to amphiphysin and thereby prevent endocytosis, attenuating the decrease in GABAA current associated with genistein. B: GABAA receptor activation in the spinal cord in normally inhibitory, where a presynaptic site of action is the primary target illustrated. In the presence of inflammation, there is an increase in both synaptic and extrasynaptic GABAA receptors. However, to account for our previous observation (Anseloni and Gold 2008) as well as those of others (Knabl et al. 2008; Witschi et al. 2011) that benzodiazepines retain their analgesic efficacy in the presence of inflammation, we hypothesize that the pronociceptive actions of GABA are due to activity at extrasynaptic GABAA receptors that are coupled to a relatively depolarized Cl− equilibrium potential. These receptors are targeted by the low dose of muscimol used in the present study. A decrease in GABAA current density via genistein eliminates the pronociceptive actions of muscimol, leaving only a residual antinociceptive activity as synaptic receptors.
