Figure 5.
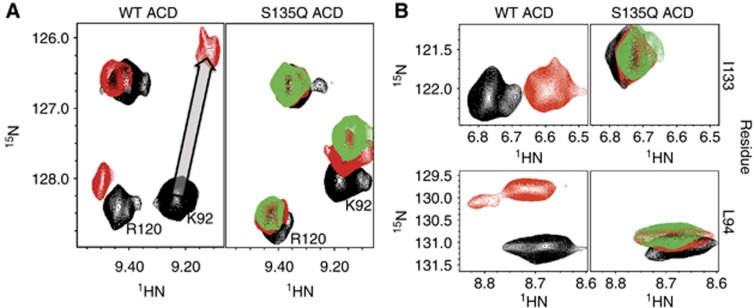
Mutation of residue S135 in the β4/β8 groove disrupts IxI peptide binding. (A) Selected resonances from 1H–15N HSQC of WT ACD (left) and the mutant S135Q ACD (right) in the absence and presence of αB-IxI peptide are compared. Spectra collected in the absence of peptide are shown in black. Spectra collected in the presence of 6-fold αB-IxI peptide (red) for the WT and S135Q ACD and 10-fold (green) peptide for S135Q are shown. In the S135Q mutant, residues K92 from the β4 strand and R120 (which is close to and behaves similarly to perturbed residues on the β3 strand) show a decrease in peptide-dependent chemical shift perturbation. The titration vector for the resonance of K92 in the WT spectra is shown as a grey arrow. (B) Peptide-induced CSPs observed for resonances of L94 and I133 are compared, with the same conditions and colouring as in (A).
