Figure 6.
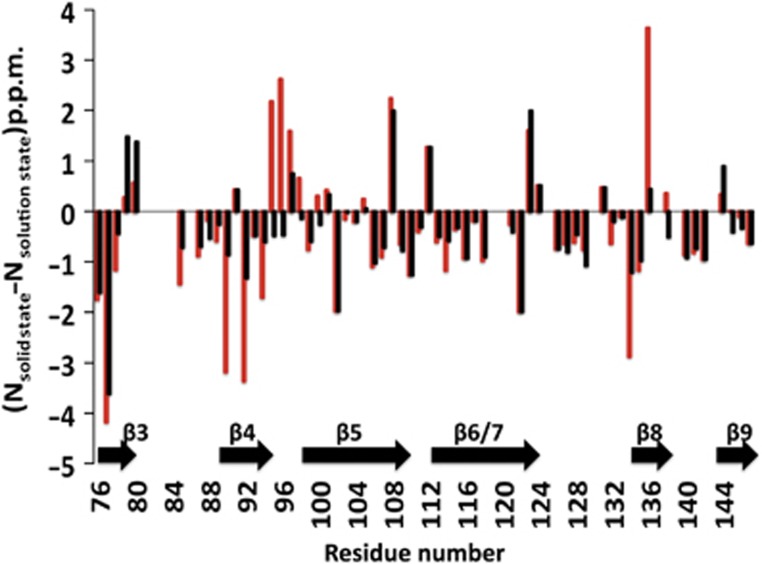
Comparison of 15N chemical shift between the αB-ACD in the context of the oligomer observed by ssNMR and isolated αB-ACD in solution. (15Nsolid state−15Nsolution state) differences were measured using the solution-state values observed both in the absence of peptide (red bars) and in the presence of saturating αB-IxI peptide (black bars). Note the greatly reduced values for (15Nsolid state-15Nsolution state) in the presence of peptide in the β4 strand and β4/5 loop (residues 89–98) and the β8 strand (residues 133–137). Positions where (15Nsolid state−15Nsolution state) could not be determined are assigned the value of 0.0 p.p.m. for clarity. For residues where multiple states were observed in the peptide saturated spectrum, the position of the most intense peak in the saturated spectrum was used.
