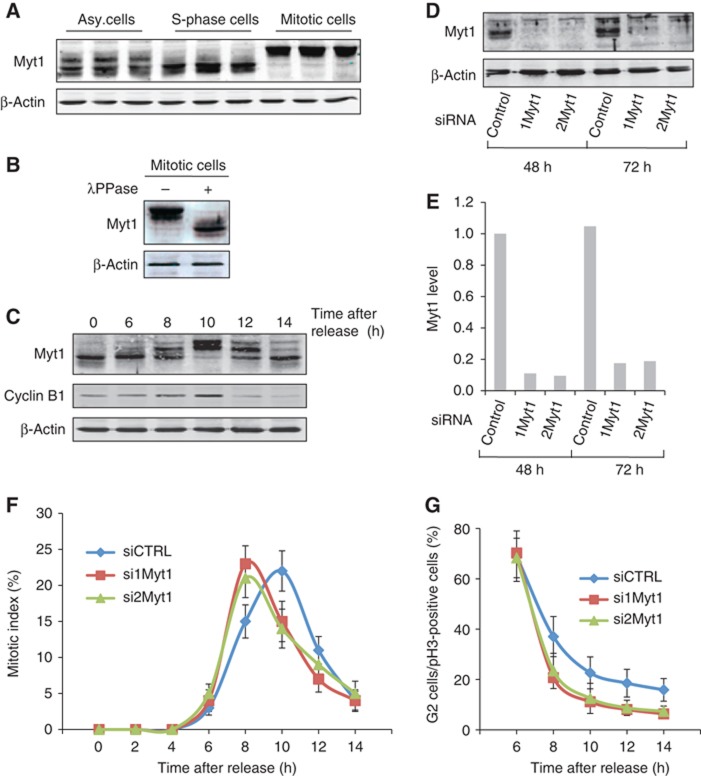
Figure 1.
Myt1 knockdown promotes early entry into mitosis. (A) HeLa cells were incubated for 18 h with DMSO (asynchronized cells), thymidine (S-phase cells) or nocodazole (mitotic cells), and Myt1 expression was analysed by western blotting the total cell lysate. Western blotting with an anti-β-actin antibody was used as a loading control. (B) Lysates from cells synchronized in mitosis were treated with or without λ protein phosphatase and analysed by western blotting with an anti-Myt1 and an anti-β-actin antibody, respectively. (C) HeLa cells were arrested in S-phase with a double thymidine block, the cells were washed to remove thymidine and at the indicated times the total cell lysates were western blotted with an anti-Myt1 antibody. Western blotting with an anti-β-actin antibody was used as a loading control and western blotting with an anti-cyclin-B1 antibody was used to monitor the progression of cells into mitosis. (D) HeLa cells were transfected with control or Myt1 specific siRNA oligos and after 48 and 72 h, respectively, the levels of Myt1 were analysed by western blotting the total cell lysates. Western blotting with an anti-β-actin antibody was used as a loading control. (E) Quantitation of Myt1 protein upon siRNA transfection. (F) The percentage of cells in mitosis (mitotic index) was determined by staining DNA with DAPI and an anti-phospho-histone H3 antibody at the indicated times after thymidine release for control and Myt1 siRNA transfected cells. 400 cells were counted for each time point (mean±s.d., n=3). (G) The percentage of cells in late G2 in the total number of cells in late G2 and all stages of mitosis was determined at the indicated times after thymidine release for control and Myt1 siRNA transfected cells. For each time point, 400 cells were counted (mean±s.d., n=3).
Source data for this figure is available on the online supplementary information page.