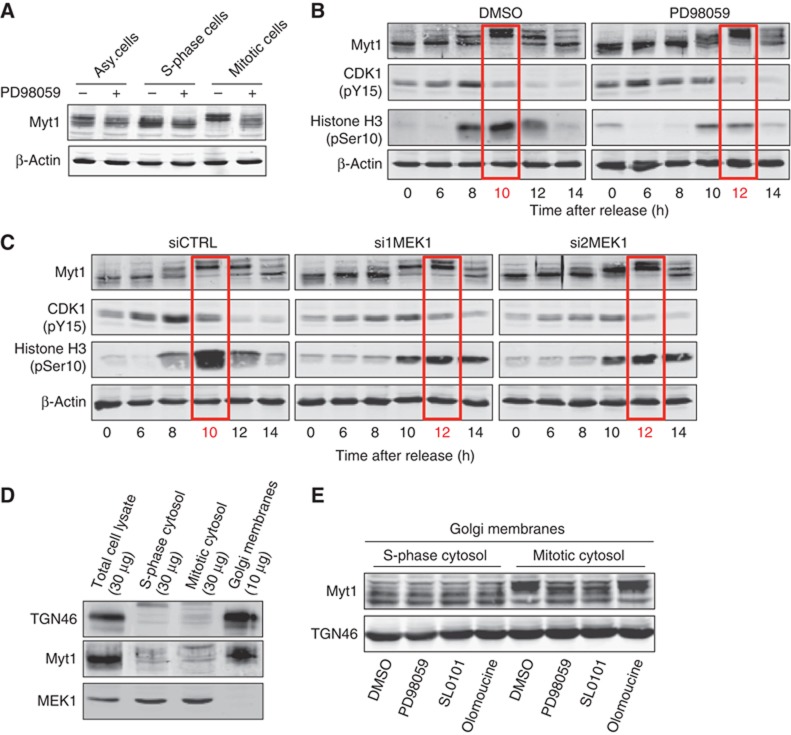
Figure 8.
MEK1 is required for Myt1 phosphorylation. (A) HeLa cells were incubated 18 h with DMSO (asynchronized cells), thymidine (S-phase cells) or nocodazole (mitotic cells), with or without PD, and Myt1 phosphorylation monitored by western blotting the total cell lysates. Western blotting with an anti-β-actin antibody was used as a loading control. (B) HeLa cells were arrested in S-phase with double thymidine block, washed to remove thymidine, and incubated with DMSO or PD. At the indicated times, the cell lysates were western blotted with antibodies to Myt1, CDK1 (pY15), Histone H3 (pSer10) and β-actin. (C) HeLa cells transfected with control or MEK1 specific siRNA oligos were arrested in S-phase with double thymidine block, washed to remove thymidine, and at the indicated times, the cell lysates were western blotted with antibodies to Myt1, CDK1 (pY15), Histone H3 (pSer10) and β-actin. (D) Total cell lysate, S-phase and mitotic cytosol, and Golgi membrane preparation was western blotted with antibodies to TGN46, Myt1 and MEK1. (E) Isolated Golgi membranes were incubated for 1 h at 37 °C with S-phase or mitotic cytosol preincubated with DMSO, PD, SL0101 or olomoucine, and an ATP-regenerating system. The reaction was terminated by the addition of an excess of cold KHM buffer supplemented with phosphatase inhibitors. The Golgi membranes were collected by ultracentrifugation, resuspended in the SDS sample buffer and western blotted with an anti-Myt1 antibody. Western blotting of the same preparation with an anti-TGN46 antibody was used as a loading control.
Source data for this figure is available on the online supplementary information page.