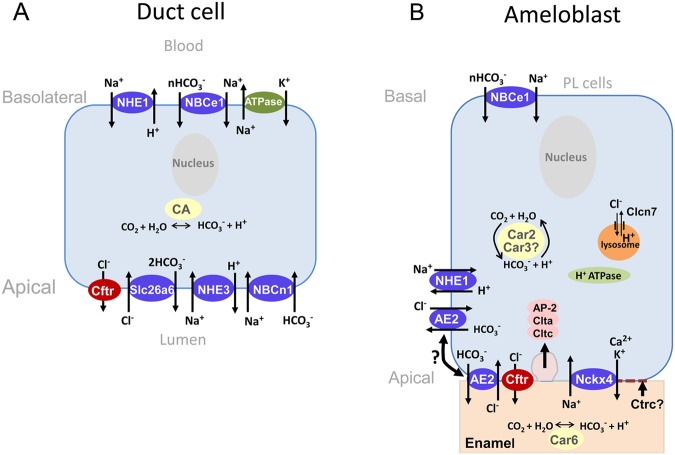
Figure 1.
Schematic representation of transepithelial transporters in pancreatic duct cells and ameloblasts. (A) Pancreatic duct cell. The main transepithelial transporters regulating pH via HCO3- movement, exhibited above, are modified from Park and Lee (2012). At the basolateral pole, HCO3- is regulated by NBCe1. Sodium uptake is mediated by NHE1, whereas extrusion involves Na+/K+ ATPases. Non-voltage-gated Na+ channels may also contribute to Na+ absorption at the basolateral pole (not shown). The apical pole of these cells expresses the Na+/HCO3- co-transporter (NBCn1) and the Na+/H+ exchanger (NHE3) that regulate Na+ intake, whereas Cl- is extruded by Cftr and exchanged by Slc26a6 for HCO3-. (B) Maturation-stage ameloblast. This model is not restricted to HCO3- transport but depicts our current knowledge of the proteins associated with the main activities of ameloblasts during maturation. Our model has been constructed based on previous reports (Bronckers et al., 2010; Josephsen et al., 2010) together with our own data. In this scheme, Ca2+ may be transported out of the cells at the apical pole by at least one of the NCKX members of sodium-calcium-potassium exchangers (NCKX4) (Hu et al., 2012). Cl- may be transported into the lysosomal system by Clcn7 to acidify luminal pH (Lacruz et al., 2013). We base our diagram on an osteoclast model (Jentsch, 2007) that commonly includes H+ATPases in the endosomal-lysosomal system. These pumps, as well as the function of a variety of CLC transporters, are ubiquitous features of endosomes-lysosomes required for luminal acidification (Jentsch, 2007). Chloride is also transported at the plasma membrane by Cftr and may also be linked to the anion exchanger AE2, as has been proposed (Bronckers et al., 2010). Two versions have been reported for the localization of AE2. In the schematic, AE2 localization at the apical pole was reported by Paine et al. (2008), whereas AE2 in the basolateral pole was described by Lyaruu et al. (2008) and Bronckers et al. (2009). Carbonic anhydrases 2 and 6 (Car2, Car6) remain important sources of localized bicarbonate production, as described elsewhere (Lacruz et al., 2012b). We hypothesize that, in addition, the isoform Car3 may also contribute to this function, which illustrates increased mRNA expression levels during maturation (Lacruz et al., 2012b). Intracellular passage of bicarbonate appears to be mediated by NBCe1 at the basal pole (Lacruz et al., 2010b). Such buffering may be required for ameliorating H+ release during Car2 and Car3 activities in the cytosol. Perhaps some of these H+ are shunted to the enamel area and others are used by the H+ATPase pumps of the endosome-lysosome. Extracellular bicarbonate transport to the enamel area may also be mediated by Cftr and AE2. In addition, as discussed in the text, if future research shows the presence of a Na+,K+-ATPase pump in ameloblasts, this would explain Na+ export to some extent. Endocytotic functions appear to be mediated, at least in part, by the AP-2/clathrin-dependent pathway in maturation-stage ameloblasts (Lacruz et al., 2013). It may also be the case that either the protease Ctrc functions in association with Klk4 in the processing of matrix breakdown which may subsequently undergo endocytosis, or it is perhaps directly associated with the lysosomal/endosomal apparatus. Note: The current diagram should be viewed as work in progress.