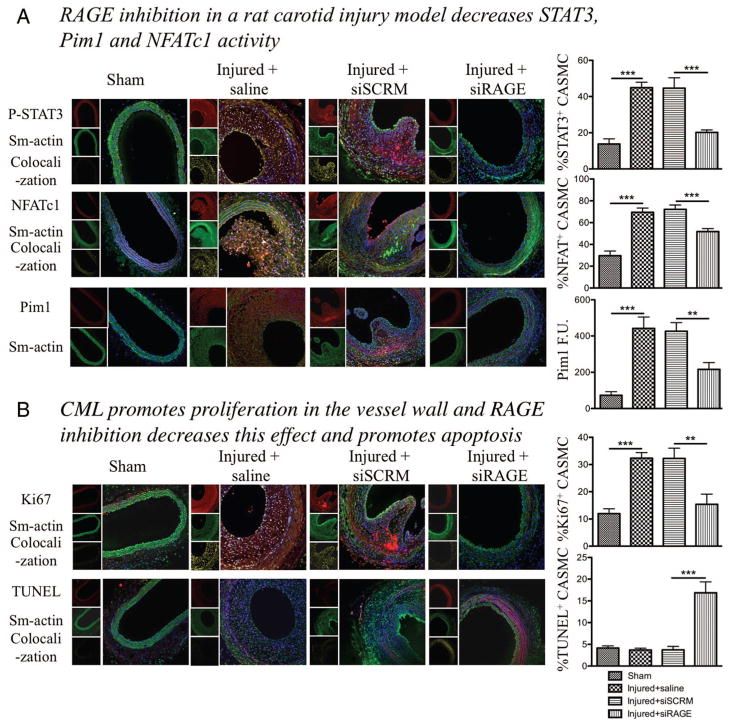
Figure 4.
Nε-(carboxymethyl)lysine-bovine serum albumin (CML-BSA) injected in rats stimulates the STAT3/Pim1/NFATc1 pathway and enhances vascular smooth muscle cells proliferation. A, CML-BSA promotes STAT3 activation through advanced glycation endproducts receptor (RAGE) in the vascular wall (immunofluorescence, n=5 rats per group, P<0.001). STAT3 activation correlates with nuclear factor of activated T-cells (NFAT) c1 activation (immunofluorescence, n=5 rats per group, P<0.001). Both STAT3 and NFAT are transcription factors and are present in the nucleus when activated. Thus, colocalization between either P-STAT3 or NFATc1 (red) and the nucleus blue, is showed by a yellow pattern, which is decreased when carotids were treated with RAGE siRNA. Pim1 expression is also enhanced in injured carotid and is inhibited when RAGE is blocked. Pim1 quantification was measured by fluorescence quantification (immunofluorescence, n=5 rats per group, P<0.01). B, RAGE/STAT3/Pim1/NFATc1 axis is responsible of cell proliferation, measured by Ki67 (immunofluorescence, n=5 rats per group, P<0.01). RAGE inhibition increases apoptosis in the vessel wall, measured by TUNEL (immunofluorescence, n=5 rats per group, P<0.01). For all staining, to calculate percentage of activation or proliferation, cells that had positive staining in nucleus were considered positive, which was divided by the total amount of cell (counted with DAPI). Again, yellow pattern shows colocalization between proliferation and apotosis factors (red) and the nucleus (blue).