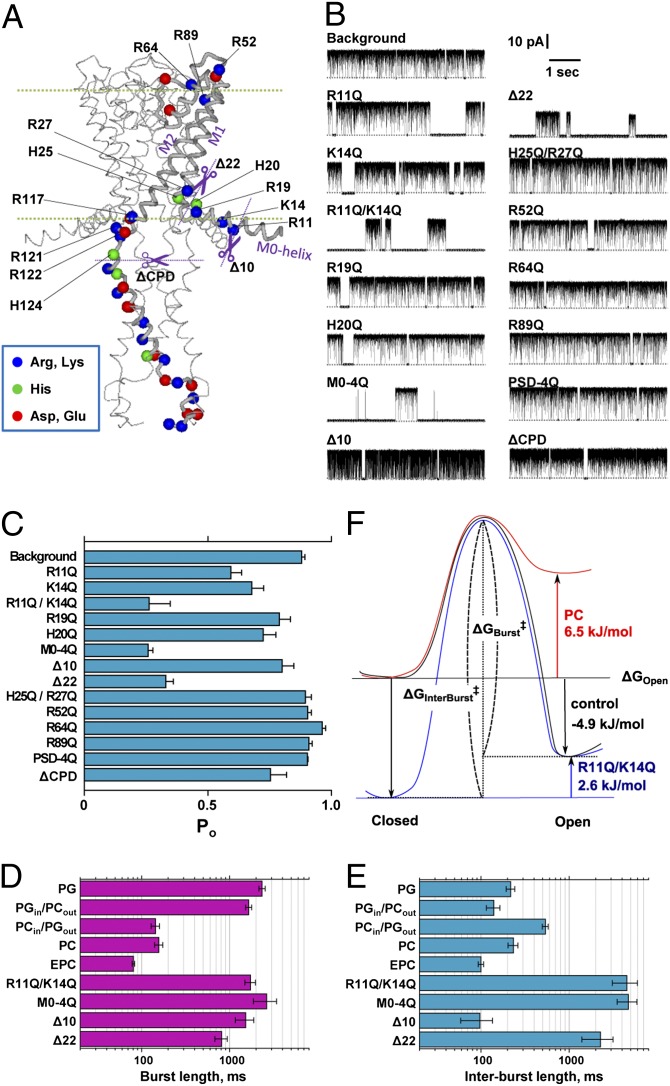
Fig. 3.
Effects of charge neutralization and the deletion mutations on single-channel currents of the E71A mutant channel. (A) Location of the charged amino acid residues in the KcsA channel. For the KcsA mutant (E71A), these charged residues were replaced with glutamine, or the N terminal or the cytoplasmic domain was truncated (purple dotted lines). (B) Representative single-channel current recordings of these mutant channels. Current traces were recorded at 100 mV in the symmetrical PG membrane. (C) Open probability of the mutant KcsA channels. Here, the background represents the KcsA (E71A); M0-4Q indicates the replacement of Arg-11, Lys-14, Arg-19, and His-20 to Gln; PSD-4Q indicates the replacement of Arg-117, -121, -122, and His-124 to Gln; ∆CPD indicates the deletion of the cytoplasmic domain (125–160) by chymotrypsin; ∆10 indicates the deletion of N-terminal 9 amino acids (2–10 aa); ∆22 indicates the deletion of N-terminal 21 amino acids (2–22 aa). Error bars represent SEM (n = 3–9). (D and E) Kinetic analyses of the burst (D) and interburst length (E). Error bars represent ±SEM (n = 3–6). (F) Hypothetical energy profiles for the closed and open transition states. Free energy level of the open state relative to the closed state (ΔGOpen) was calculated from the popen values, and the barrier height or the activation energy (ΔG‡: ΔGBurst‡ and ΔGInterBurst‡) was calculated using the rate constants of the transitions (the reciprocals of the mean burst length and mean interburst length). ΔGBurst‡ was 77.5 kJ/mol, 68.9 kJ/mol, and 74.8 kJ/mol for PG, PC, and R11Q/K14Q. ΔGInterBurst‡ was 69.7 kJ/mol, 69.8 kJ/mol, and 77.4 kJ/mol for PG, PC, and R11Q/K14Q.