Fig. 5.
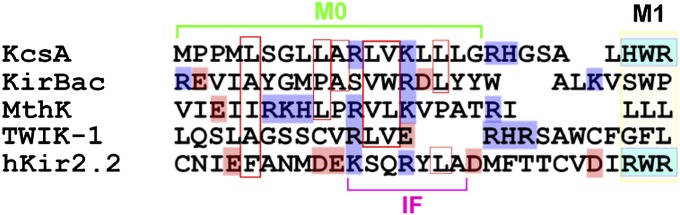
Alignment of the amino acid sequence of the N-terminal region of the KcsA and two-transmembrane channels. Amino acids having a net charge are depicted in color (Right): blue, positive; pink, negative. Numbers indicate the amino acid 28 sequence of KcsA. IF represents the interfacial helix (numbers 61–69) assigned from the crystal structure of Kir2.2 [Protein Data Bank, (PDB) ID code 3SPI]. IF helix corresponds to the C-terminal half of M0 (numbers 11–18), and R65 in the IF helix (corresponding to K14 in KcsA) interacts with the tether helix in the cytoplasmic domain (number 16).
